Home mechanical ventilation (HMV) is a prevalent therapy (6.6–12.0 per 100,000 inhabitants1–3) for chronic respiratory failure patients. Only in the Organization for Economic Co-operation and Development countries, ≈125,000 patients are already receiving HMV (for a prevalence ≈10 per 100,000 population and ≈1.25 billion inhabitants). Remarkably, HMV users are expected to further increase owing to the obesity epidemics, the ageing of population4 and the expected HMV raise in highly populated Asia-Pacific countries.5
HMV is complex since it requires that a Class II active medical device is placed at the patient's home for very a long term (usually for life). Contrary to conventional mechanical ventilation provided within health care premises, HMV is applied with no direct and constant supervision of health care staff. In fact, despite these professionals are responsible for the patients through hospital and home visits, HMV treatment is usually managed by an external health care provider which is in charge of placing the ventilators at the patient's home, of ensuring that the device is set according to the ventilation parameters prescribed by the attending physician and of periodically revising and technically servicing the ventilator. Such a complex, long-term health care provision pathway is subjected to potential dysfunctions, thereby requiring well-established quality assurance procedures.6
We here report the results of a current quality assessment analysis on the HMV provided to 147 patients attended by 5 university hospitals from 3 different regional public health care systems in Spain: Clinic-Barcelona (54%), Vall Hebron-Barcelona (7%), Sant Pau-Barcelona (7%), Universitario Marques Valdecilla-Santander (22%) and Universitario-Burgos (10%). Patients (56% women) were randomly selected from those attending an outpatient routine visit to follow-up their bilevel pressure HMV, suffered from neuromuscular diseases (29%), lung and airway diseases (38%), thoracic cage abnormalities (18%) and obesity hypoventilation syndrome (15%). The mean prescribed inspiratory pressure (Pins) and expiratory pressure (Pexp) were 16.7±3.8 (9-25) cmH2O and 6.7±2.1 (4-14) cmH2O, respectively (mean±SD (range)). Performance of the study was not communicated to the companies providing HMV to avoid any non-routine servicing. To dissect potential sources of dysfunction, the following Pins and Pexp values were recorded: those prescribed by the physician as indicated at the patient's clinical record, those set at the control panel of the ventilator and those actually delivered by the ventilator when it was applied to a patient simulator. The ventilator, with the very same settings as in each patient's home, was connected to a commercially available portable testing device (CITREX-H4, Imtmedical, Switzerland) consisting of a computerized sensors unit (including pressure and flow sensors) and a passive (i.e. no simulated breathing efforts) resistance (R)–compliance (C) lung model with different impedances (R=5cmH2Os/l, C=30ml/cmH2O), (R=20cmH2Os/l, C=30ml/cmH2O) and (R=5cmH2Os/l, C=10ml/cmH2O) labelled as normal, obstructive and restrictive, respectively. These R-C combinations were not aimed at representing the typical patient of each category, but rather considerably high respiratory impedances to test the devices under very demanding conditions.7 The testing device (with its pressure sensor calibration checked each measuring session), was placed between the nasal/face mask and the lung model. For these 3 patient models, measurements were carried with/out including an orifice-type air-leak (30l/min at 10cmH2O) at the mask level to simulate real-life lack of perfect fitting between mask and patient's face.7 This simulated “unintended leak” was placed in addition to the intended leak in the HMV equipment. The differences observed in Pins and Pexp when comparing the values prescribed, set at the ventilator and effectively delivered by the ventilator were subjected to Bland–Altman analysis to compute the bias and limits of agreement as the mean and±1.96 SD of the differences between values, respectively.8 Measurements on all ventilators were carried out by a unique physiotherapist expert in non-invasive ventilation and the same testing device was used for all ventilators.
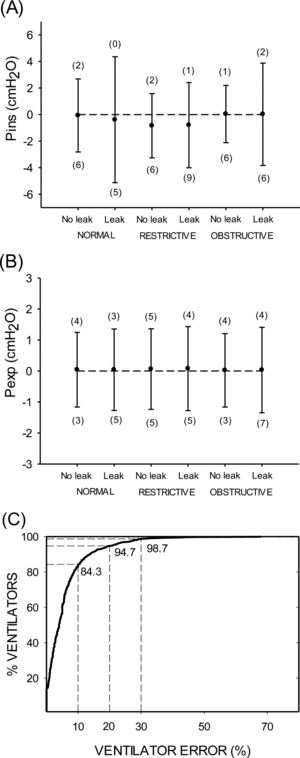
Fig. 1 shows the results corresponding to the difference between the values actually delivered by the ventilator and the ones prescribed by the physician, thereby assessing the whole delivering pathway of HMV provision. For any of the 6 combinations of patient models with/out air-leak, the bias for Pins was quite low (−0.84cmH2O in the worst case, which corresponded to the restrictive model). However, the limits of agreement for Pins ranged considerably (−5.14, 1.57cmH2O) among the different conditions. There was no significant linear correlation between the differences observed in Pins and the absolute value of Pins. By contrast, Pexp exhibited a negligible bias (always less than 0.1cmH2O) and small limits of agreement (−1.1, 1.4cmH2O). Interestingly, the number of ventilators outside the limits of agreement (for the different patient models with/out air-leak) were a maximum of 9 (6.1%) and of 7 (4.8%) out 147 devices for Pins and Pexp, respectively (Fig. 1A and B). Accordingly, most ventilators were within the Bland–Altman limits of agreement. As expected, the limits of agreement were wider in case of superimposing an air-leak regardless of patient model, particularly for Pins (Fig. 1A). Given that Pins showed higher variability and that it is the most relevant parameter in bilevel pressure HMV, an error index of Pins was computed for each ventilator, defined as the positive value of the percentage difference between the actual Pins yielded by the ventilator (for all patient models with/out air leak) and the prescribed value.9 As shown by Fig. 1C, the cumulative percentage of ventilators as a function of the ventilator error indicates that this error was lower than 10%, 20% and 30% in 84.3%, 94.7% and 98.7% of ventilators. Although these percentage could seem low from a statistical viewpoint, it should be kept in mind that from a quality control perspective each single device (and thus treated patient) is important.
(A) Comparison of the inspiratory pressure (Pins) actually delivered by ventilators and the values prescribed by the attending physician when the ventilator was applied to different patient models (normal, restrictive and obstructive diseases) with/out an air-leak (see text for explanation). Data are bias±limits of agreement in Bland–Altman analysis. Figures in brackets indicate the number of ventilators (out of 147) which are beyond the upper and lower limits of agreement in the Bland–Altman analysis. (B) Same as (A) for expiratory pressure (Pexp). (C) Cumulative percentage of ventilators as a function of the error index of Pins in the ventilator (see text for details).
A possible cause of the observed differences between prescribed values and delivered HMV could be caused by an incorrect translation of the ventilation settings prescribed by the physician into the control panel of the ventilator. However, this cause of error was relatively low since Bland–Altman comparison of Pins in the clinical record and in the ventilator control panel resulted in negligible bias (−0.07cmH2O) and limits of agreement (−0.94, 0.81cmH2O) were much lower than those in Fig. 1. Similar findings were found for Pexp. Accordingly, the main differences observed in Pins should be attributed to the fact that ventilators are not always able to deliver the target values.10–13 Although the current results indicate that improvement in HMV provision is still required, it is interesting to note that dysfunction in the current provision of HMV reported here is clearly lower than that reported 14 years ago when a similar study was carried out in hospitals of the very same health care system.9 In particular, it is remarkable that the errors in translating the prescribed settings to the ventilator control panel have been considerably reduced in bias (from −0.23 to −0.07cmH2O), and particularly in limits of agreements (64% reduction: from −2.77, 2.32 to −0.94, 0.81cmH2O).
In conclusion, notwithstanding the limitations of using a simplified patient modelling, current data on real-life HMV application suggest that it has been a clear quality improvement in the provision of this therapy. However, a continuous effort is required to establish and maintain quality assessment procedures, particularly considering that this complex therapy pathway will be progressively prescribed by hospitals with limited experience in the follow-up of such an externalized treatment. Extensive use of ventilators allowing daily telemonitoring ventilation variables could help in the quality assessment of HMV.14,15
FundingThis work was supported in part by Spanish Ministry of Economy and Competitiveness PI17/01068 and SOCAP.
The authors want to thank Cristina Embid and Monica Matute for their help in data collection. The authors also thank Maria Dolores Nuñez, Juan Jose Hernandez Diaz, Pedro A Anton Albisu for his help and Albert Gabarrus for his statistical support.