Atrial fibrillation (AF) is the most common supraventricular tachyarrhythmia, and the arrhythmia that causes most morbidity and mortality. More than 90% of the underlying ectopic foci of electrical activity originate in the pulmonary veins (47% in the left superior pulmonary vein).1
In recent years, a rhythm control strategy has been developed in which the pulmonary veins are isolated electrically with catheter ablation. This procedure is indicated in the first-line treatment of patients with symptomatic paroxysmal AF refractory to antiarrhythmic drugs, and offers improved quality of life with a complication rate of 2.9%.2
We present a case of pulmonary vein occlusion and stenosis with venous infarction as a complication of ablation for AF.
Our patient was a 61-year-old hypertensive man, who had undergone AF radiofrequency ablation 4 months previously. He presented with dyspnea and sudden onset of intense left pleuritic pain, with no symptoms of nausea, vomiting, or sweating, and no fever. The patient also complained of cough with mild bloody expectoration.
On examination, breathing was normal at rest, and no signs of hemodynamic compromise were observed. Auscultation revealed reduced breath sounds in the left lung base.
Oxygen saturation in room air was 96%, and D-dimer concentration was normal (0.29mg/L).
Chest X-ray revealed peripheral alveolar consolidation in the lower lateral region of the left upper lobe (LUL) and lingula, associated with pleural effusion (not shown).
In view of the clinical picture (bloody sputum) and radiological findings in the chest, and despite the normal D-dimer results, we felt it necessary to request a chest CT angiogram to rule out pulmonary thromboembolism.
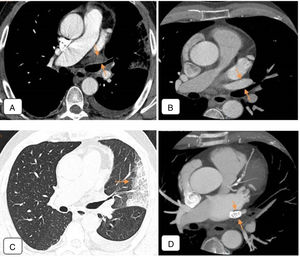
This examination revealed total occlusion of the left superior pulmonary vein, with the lumen occupied by a material that showed mild enhancement (Fig. 1A). Severe stenosis was also detected in the left inferior pulmonary vein (not shown).
(A) Chest CT angiogram 4 months after ablation, showing total occlusion of the left superior pulmonary vein, the lumen of which is occupied by material that shows mild enhancement (arrows), that probably corresponds to inflammatory tissue. Moderate-sized left pleural effusion. (B) Pre-ablation chest CT angiogram. Axial image of a region similar to that of (A). Left superior pulmonary vein is permeable with normal caliber (arrows). (C) Chest CT of pulmonary parenchymal window: alveolar consolidation in left upper lobe and lingula, coinciding with the drainage territory of the obstructed left superior pulmonary vein, consistent with venous infarction (arrow). (D) Follow-up CT angiogram after angioplasty. Stent restoring patency observed in left superior pulmonary vein (arrows).
In the pre-ablation CT angiogram, the caliber of the left superior pulmonary vein was normal and the lumen was permeable (Fig. 1B).
In the post-ablation lung window image, alveolar consolidations were observed in the lower lateral region of the LUL, lingula, and posterior base of the lower lobe. These consolidations coincided with the drainage of the obstructed, stenotic veins seen on the CT angiogram, and, as such, were diagnosed as venous infarction (Fig. 1C).
Cardiac catheterization was subsequently performed, confirming occlusion of the left superior pulmonary vein and critical stenosis of the left inferior pulmonary vein at the level of the ostium. Balloon angioplasty, followed by stent implantation in each vein, were performed in the same procedure (Fig. 1D).
Non-invasive imaging techniques are used during pulmonary vein ablation, both for planning and for guidance during the procedure.2 Echocardiography is the technique of choice for the evaluation of congenital heart disease, but it is less than optimal for evaluating the cavo-atrial junction and inappropriate for visualizing the more proximal parts of the pulmonary veins. The most appropriate techniques for defining pulmonary vein morphology and size, and for obtaining reference baseline images for subsequent evaluations of acute or late complications, are 3D gadolinium-enhanced magnetic resonance angiogram and contrast-enhanced CT angiogram (cardiac gating is not necessary).1
The anatomy of the pulmonary veins and their possible anatomical variants must be understood. Under normal conditions, 4 pulmonary veins carry oxygenated blood from both lungs and drain into the left atrium. The right superior pulmonary vein drains the upper and middle lobes, the left superior pulmonary vein drains the upper lobe and lingula, and the 2 inferior pulmonary veins drain the lower lobes.3 In the distal segment of the pulmonary veins, a 2–17mm section passes through the myocardium (myocardial sleeves), and this is a common site of ectopic electrical activity.4
The major congenital variants include an abnormal number or diameter of pulmonary veins, abnormal drainage, and abnormal connections with the pulmonary arterial tree. Acquired abnormalities include hypertension, thrombosis, calcifications, collateral circulation, and stenosis or obstruction. The latter 2 may be caused by cancer, fibrosing mediastinitis, tuberculosis, or complications after radiofrequency ablation.3
Complications arising from the ablative procedure are caused by thermal injury to the vessel wall.5 Pulmonary vein stenosis occurs in 0.5% of patients, and usually develops about 3 months after ablation.2 Thermal lesions produce scarring and contraction of the vessel wall, causing architectural remodeling and hyperplasia of the intima, producing venous stenosis. Patients may have non-specific respiratory symptoms (dyspnea, cough, chest pain, or hemoptysis) and the severity of symptoms is associated with the number of veins affected, and the degree, length, and duration of stenosis.5,6
Mild stenoses may be difficult to detect. CT angiogram clearly shows pulmonary venous occlusion, but this complication is rarer, since anticoagulation starts immediately after the procedure.2
Pulmonary parenchymal abnormalities are indirect signs of significant stenosis or venous occlusion, which can include multifocal opacities or peripheral consolidations that might indicate alveolar infarction or hemorrhage, or interstitial septal thickening.5 Venous occlusion is frequently accompanied by perivenous infiltrate and locoregional lymphadenopathies caused by thermal damage.2
Stenoses are managed according to the grade of severity compared to the pre-ablation findings. If stenosis is 50%–70%, follow-up in 3–6 months is recommended; if stenosis is 75%, a repeat CT in 3 months is recommended, and if it is 90%, urgent treatment is required within 3–6 weeks, since the lesion could progress. The treatment of choice in this case is angioplasty followed, if necessary, by stent placement.2
In conclusion, pulmonary vein stenosis and occlusion are increasingly rare complications of ablation for AF, but it is important that they are investigated, since a good prognosis depends on early diagnosis and prompt treatment. Imaging techniques such as CT angiography play a fundamental role in the management of these lesions, thanks to their good anatomical resolution, rapid results, and availability. In-depth understanding of the anatomy of the pulmonary veins and radiological findings of the complications of ablative surgery are therefore essential.
Please cite this article as: Fernández-Navarro L, Moya-Sánchez E, Segura-Rodríguez D, Ruiz-Carazo E. Oclusión venosa pulmonar como complicación del tratamiento ablativo de la fibrilación auricular. Arch Bronconeumol. 2018;54:338–340.