More than 2 billion individuals are estimated to be infected with Mycobacterium tuberculosis.1 The primary objective of antituberculosis treatment is to rapidly eliminate bacilli and prevent the development of resistance. Greater effectiveness has been achieved when first-line regimens of anti-tuberculosis drugs are administered for 6 months.2 Drug resistance, contraindications, or intolerance may require first-line drugs to be replaced by 1 or more second-line agents.2 We report the case of a patient with tuberculosis (TB) and a history of celiac disease (CD) who was treated with first-line anti-TB drugs that were not tolerated, prompting a switch to a second-line agent, resulting in good progress.
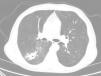
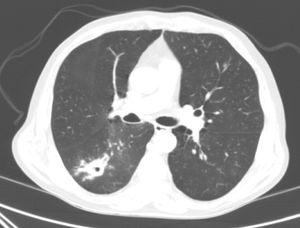
Our patient was a 70-year-old man, with a history of active smoking, diabetes mellitus, ischemic heart disease, and CD. He was admitted in June 2016 with a 2-day history of hemoptysis. Chest radiograph revealed an opacity in the right lower lobe (RLL). The chest computed tomography (CT) revealed a poorly defined pseudonodular cavitary lesion, measuring 35mm in its longest diameter, in an upper segment of the RLL, and multiple adjacent centrilobular and ground glass opacities (Fig. 1). No diagnosis was reached with either fiberoptic bronchoscopy or CT-guided needle biopsy of the lesion, so a PET-CT was performed, showing significant pathological uptake. An intraoperative biopsy was then performed, and the pathology study reported a diagnosis consistent with a chronic inflammatory necrotizing granulomatous process, probably due to TB. Unfortunately, the sample was not submitted for microbiological study, and the patient was diagnosed with probable TB.
In August 2016, treatment began with anti-TB drugs (isoniazid, rifampicin, pyrazinamide, and ethambutol combined in the same tablet – Rimstar® – a gluten-free preparation). Tolerance was initially good. One month after starting treatment, the patient was admitted for diarrhea (10–15 bowel movements a day) and 5kg weight loss. Clinical laboratory tests showed secondary metabolic acidosis. Stool samples were negative for Clostridium difficile, and no enteropathogens or parasites were isolated. The patient's family members reported that he did not adhere to his gluten-free diet, so the same antituberculous drugs were administered in individual preparations while following the correct diet. IgA anti-transglutaminase antibody detection was requested (a marker for dietary transgression), which was negative, and treatment began with metronidazole. This reduced the number of bowel movements and the patient was discharged. Three days later he was admitted again due to persistent diarrhea, decline in general condition, and metabolic acidosis. In view of these findings, tuberculostatic treatment was discontinued. This led to resolution of the diarrhea and other abnormal laboratory test results. We reviewed the ingredients of the medications and found that some them (specifically isoniazid) contained gluten (wheat starch). Gastroscopy showed subtotal villous atrophy (Marsh 3c), and no changes were observed on colonoscopy. Finally, the patient was discharged again without tuberculostatic treatment.
One month later, he attended a follow-up visit and reported no episodes of diarrhea and 6kg weight gain. On the assumption that the lesion was paucibacillary, we decided to treat the patient with 2 drugs for 9 months. The most important contribution of isoniazid to the treatment of TB is known to be its high bactericidal activity. However, when sputum smear, culture, and bronchial aspirate are all negative, as was the case in our patient, the bactericidal role of isoniazid becomes of less importance, since in these cases sterilizing drugs, such as rifampicin and moxifloxacin, play a more decisive role.3,4 The only component, then, of the 4-drug combination (that had been so poorly tolerated twice previously) that was really important for our patient's form of TB was rifampicin. Rifampicin was reintroduced at escalating doses (first day, 100mg; second day, 200mg; third day, 300mg; fourth day, 400mg; fifth day, 500mg; and sixth day, 600mg). Good tolerance was confirmed, and moxifloxacin was added 1 week later (the first 3 days, 200mg and 400mg thereafter). After confirming rifampicin tolerance, we were keen to add a new drug, that in addition to good bactericidal activity, also had a sterilizing effect. The ideal choice in this case was moxifloxacin. In subsequent visits, the patient reported good tolerance to the drugs, and no changes were detected on clinical laboratory tests.
Some systemic diseases increase the risk of developing active TB, and CD is one of these risk factors.5 Although the mechanism has not been entirely clarified, it may be due to malabsorption and lack of vitamin D in individuals with this disease.6 Celiac patients often have persistent low-grade inflammation and vitamin deficiencies, even many years after the introduction of a gluten-free diet. This diet also tends to be low in vitamin D, increasing the risk of deficiency. Vitamin D has been shown to induce nitric oxide synthesis in the macrophages, suppressing intracellular M. tuberculosis growth. It also increases the effect of interferon-γ in promoting the granulomatous process, and induces the differentiation of monocytes to epithelioid cells and multinucleated giant cells that form a major part of the granulomas.6,7
The strongest genetic links with CD are found in the MHC locus, and the correlation with HLA-DQ2 (DQA1*05/DQB1*02) is well established.6 Associations between TB and various HLA alleles have been documented, but they are not as strong as in CD.6 In northern Europeans, HLA-DQ2 is often part of the 8.1 ancestral haplotype8 that contains a number of genes, including specific alleles of class I and class II HLA molecules, and genes coding for TNF-α and C2 and C4 complement factors. Since the C2 molecule is important in the mycobacterial invasion of macrophages, a C2 allele in particular may promote TB infection in a patient subgroup.6
In addition to increasing the risk of developing TB, CD may be a risk factor for TB complications, increasing severity and the risk of death (up to 6-fold) due to TB.4 An additional complication is that patients with TB who have CD-mediated malabsorption may be at risk of developing resistance to anti-TB drugs, due to their lower bioavailability.6 As in our case, drug tolerance among CD patients may also be poor, since some medications such as isoniazid are formulated with ingredients containing gluten. By replacing some these first-line drugs with a second-line drug, we were able to improve tolerance, and to continue treatment.
In conclusion, poor tolerance to TB medications in a celiac patient requires a review of the drug components to see if they contain gluten. If the problem persists after the gradual introduction of the drugs, first-line drugs can be replaced by second-line agents.
Please cite this article as: Cerezo Lajas A, Caminero Luna JA, Rodríguez Guzmán MC, de Miguel Díez J. Tratamiento antituberculoso en un paciente con enfermedad celíaca. Arch Bronconeumol. 2018;54:337–338.