Pulmonary rehabilitation (PR) has been shown to improve dyspnea, exercise capacity and health-related quality of life in patients with chronic obstructive pulmonary disease (COPD). PR has also shown benefits in diseases other than COPD but the level of evidence is lower. The fundamental components of PR programs are muscle training, education and chest physiotherapy. Occupational therapy, psychosocial support and nutritional intervention should also be considered. Home programs have been shown to be as effective as hospital therapy. The duration of rehabilitation programs should not be less than 8 weeks or 20 sessions. Early initiation of PR, even during exacerbations, has proven safe and effective. The use of oxygen or noninvasive ventilation during training is controversial and dependent on the patient's situation. At present, the best strategy for maintaining the benefits of PR in the long term is unknown. Longer PR programs or telemedicine could play a key role in extending the results obtained.
La rehabilitación respiratoria (RR) ha demostrado mejorar la disnea, la capacidad de esfuerzo y la calidad de vida relacionada con la salud en los pacientes con enfermedad pulmonar obstructiva crónica (EPOC). En otras enfermedades distintas de la EPOC también ha mostrado beneficios, aunque el grado de evidencia es menor. Los componentes fundamentales de los programas de RR son el entrenamiento muscular, la educación y la fisioterapia respiratoria, siendo aconsejable también contemplar la terapia ocupacional, el soporte psicosocial y la intervención nutricional. Los programas domiciliarios han demostrado igual eficacia que los hospitalarios. La duración de los programas de RR no debe ser inferior a 8 semanas o 20 sesiones. La RR iniciada precozmente, incluso durante las exacerbaciones, ha demostrado ser eficaz y segura. La utilización de oxígeno o ventilación no invasiva durante el entrenamiento es controvertida y dependiente de la situación del paciente. En el momento actual desconocemos cuál es la mejor estrategia para mantener los beneficios de la RR a largo plazo. Una mayor duración de los programas o la telemedicina podrían ser claves para prolongar los resultados conseguidos.
Since the SEPAR guidelines on Pulmonary Rehabilitation (PR) were published in 2000,1 the magnitude of the changes that have occurred in this discipline has been so great that we are compelled to update them in line with other scientific societies.2–4 In the years since then, we have gained greater insight into the pathophysiology of respiratory diseases, the methods of applying PR are better known,2–4 we understand the importance of early initiation of the therapy (recommending it even after an exacerbation),5 we have learned which components of a PR program are essential2–4,6 and we have achieved active patient implication through good education and the implementation of self-care and self-management programs.7 Thus, PR is now the therapy of choice in comprehensive long-term care models.8
The key components of PR programs are currently exercise, education and chest physiotherapy, while occupational therapy, psychosocial support and nutritional intervention should also be considered.2–4 However, despite the available evidence, use of PR is not widespread in Spain,9 and its implementation is far from ideal, showing wide geographical variation and above all, a considerable degree of under-use.9
In these SEPAR Guidelines, after establishing the general concepts of PR, we will discuss exercise training, patient education, chest physiotherapy, psychological and nutritional support, the specific features of PR in patients with COPD, and finally the role of PR in chronic respiratory diseases other than COPD. In relation to patients with neuromuscular diseases, international guidelines2–4 mention the importance of considering this group as candidates for PR programs especially adapted to their needs. An increasing number of such patients are treated in respiratory medicine units/departments, not only using mechanical ventilation techniques, but also from a more comprehensive perspective, with particular focus on respiratory complications, mainly retention of secretions.10 To that end, we have devoted an on-line supplement specifically to these diseases.
The GRADE system has been adopted to establish the level of evidence and the strength of the recommendations given in the guidelines.11
General ConceptsDefinitionRecent advances in the field of PR have redefined the scope of this therapy. The American Thoracic Society (ATS) and the European Respiratory Society (ERS) have defined Pulmonary Rehabilitation as: a comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education and behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors.2
Composition of the Pulmonary Rehabilitation TeamA PR team should be composed of at least one respiratory physician, one physiotherapist, one nurse trained in respiratory diseases and, if possible, one rehabilitation specialist. Ideally, the team should also include, or at least be in close contact with, a social worker, an occupational therapist and a psychologist.
The recent ATS/ERS statement2 considers that, given the multidisciplinary nature of PR, the most important factor is that the team is made up of motivated professionals with expertise in chronic respiratory diseases. The composition of the team may vary from country to country, and will mainly depend on the facilities available in each center, although no particular staffing structure has been found to be better than others.
Selection CriteriaIn order to obtain the expected benefits of PR, patients must be carefully selected; those considered to be candidates for inclusion in PR programs are patients with COPD and limiting dyspnea greater than or equal to grade 2 on the modified Medical Research Council (mMRC) scale (1A). Also considered as candidates are hypersecretory patients with cystic fibrosis or bronchiectasia (1B), patients with neuromuscular disease and ineffective cough (1C), patients requiring chest surgery (1C) and those with other chronic restrictive lung diseases such as diffuse interstitial lung disease or pulmonary hypertension (1B).2–4,12,13 International guidelines2,4 indicate that PR should be accessible to all patients with chronic respiratory disease, irrespective of their age or severity of disease, and that it is essential to tailor the program to each individual patient.
Patients not suitable for inclusion in a PR program are those with psychiatric or behavioral disorders that impair collaboration, patients with acute or unstable cardiovascular disease that limits their ability to exercise, and patients with locomotor diseases that are incompatible with exercise training.2–4
Candidate EvaluationThe pulmonologist should make an initial clinical, radiological and functional assessment of patients who are candidates for PR. If a muscle training program is to be proposed, an electrocardiogram, 6-minute walk test14 and maximal graded exercise test (either with a shuttle walking test15 or incremental ergometer test) should be requested.16 It should be remembered that patients who desaturate (SpO2≤90%) in the walk test may benefit from the use of oxygen during training17,18 (1C).
The PR team establishes the treatment plan and follow-up. If necessary, the patient should be sent for assessment by a speech therapist, nutritionist, cardiologist, rheumatologist or other appropriate specialist.
Programs and ComponentsPR programs should basically include muscle training (1A), education (1B) and chest physiotherapy (1B), while occupational therapy (2D), psychosocial support (2C) and nutritional intervention (2C) should also be considered.
Programs should last at least 8 weeks or 20 sessions, with 2–5 sessions a week2–4 (1A), although shorter programs may also be scheduled.19,20
Program LocationPR programs should be supervised (1A). They are generally carried out in a hospital setting, although similar benefits can be obtained when performed in the home.2,4,21–26
Measuring the ResultsAssessment of PR results in patients with COPD is standardized, and involves quantifying the changes in aspects of the disease can be modified by PR, namely perception of dyspnea, health related quality of life (HRQoL) and exercise capacity.
- (1)
Various scales can be used to evaluate dyspnea in activities of daily living, such as the modified MRC scale (mMRC)27 (Fig. 1a), Mahler baseline/transitional dyspnea index,28 oxygen cost diagram28 or the dyspnea area from the original chronic respiratory questionnaire (CRQ).28 The most widely used is the mMRC scale, due to its simplicity and reproducibility. In the evaluation of dyspnea on exertion, the most commonly used is the BORG scale, applied in this case before and after an exercise stress test (Fig. 1b).
- (2)
Different questionnaires can be used to evaluate HRQoL. The CRQ, either interviewer-28 or self-administered,29 measures changes in dyspnea and quality of life, with 0.5 points being considered the minimal clinically important difference.30 The Saint George Respiratory Questionnaire (SGRQ) also measures the effect of PR on the quality of life,28 with a score of 4 considered as the minimal clinically important difference.28 Other questionnaires used in PR are the generic SF36 questionnaire or its short version SF1228 and, more recently, the Chronic Obstructive Pulmonary Disease Assessment Test (CAT).31
- (3)
Changes in exercise capacity are determined on the basis of the distance walked in the 6-minute walk test,14 with 35m considered the minimal clinically significant difference, or 26m if the patient has COPD with severe obstruction.32 Alternatively, the shuttle walk test15 may be used, where the minimum change is 47.5m.33 A better, more reproducible method of assessment would be to evaluate exercise capacity in terms of tolerance time using a resistance or sub-maximal test with a cycle ergometer. Normally, a constant level of exercise is performed that represents 70%–85% of the maximum achieved in a graded exercise test. The minimal clinically significant difference is considered to be 100–105s. This test also analyzes dyspnea and minute ventilation at the same exercise level, as well as the inspiratory capacity as a reflection of dynamic hyperinflation.2
The skeletal muscles are the main therapeutic objective of PR, and muscle training programs are the only intervention that has been shown to be capable of improving peripheral muscle dysfunction in COPD. Therapeutic physical exercise involves gradually and correctly overloading muscles to induce the functional adaptations pursued. In patients with chronic respiratory diseases, general exercise training should be aimed at improving both aerobic capacity and peripheral muscle strength.2–4,34
Aerobic or Resistance TrainingThis is the most widely used and most evidence-based exercise modality in PR (1A).2–4,34 Aerobic exercise is a sub-maximal exercise involving large muscle masses and is maintained for a prolonged period. It improves muscle strength and cardiovascular response.3 Training with a cycle ergometer or treadmill are examples of aerobic exercise most widely used in PR, especially in the hospital setting and outpatient programs. There are other aerobic exercise modalities, such as walking outdoors, swimming, dancing, and Nordic walking, but in recent studies, modalities that include walking have been shown to be best if the objective is to improve the walking capacity.2–4 Some of these modes of aerobic exercise have the advantage that they may be done outside the PR hospital unit, in the patient's home, and as such are highly recommended for the maintenance phase of programs and for exclusively home-based protocols.34 Aerobic exercise should generally be performed at least 3 times a week for 20–30min continuously or at intervals, the latter being particularly recommended for more symptomatic patients. The intensity of exercise is very important in the prescription of therapeutic exercise. High levels are known to result in a greater physiological response, so a working intensity ranging from 60% to 80% of the maximal exercise capacity (evaluated previously by means of an exercise test) is recommended. With respect to the total duration of training, a minimum of 8 weeks or 20 sessions is recommended.2–4,34
Interval Training2,4This is an adaptation of standard aerobic training in which short periods (lasting 1 or 2min) of high intensity exercise are alternated regularly with equally short periods of rest or lower intensity exercise. Patients thus achieve high levels of exercise, but with less dyspnea and fatigue, and obtain benefits equivalent to those of classic aerobic training.2–4 As previously mentioned, this adaptation is particularly recommended for more symptomatic or disabled patients who cannot maintain periods of continuous exercise.
Strength TrainingFollowing the “principle of specificity”, muscle strength training can increase the strength and mass of the muscles exercised. The evidence available supports the use of strength training combined with general aerobic training (1A), as it further increases peripheral muscle strength.2–4 In addition to improving muscle function, strength training can help maintain or increase bone mineral density levels in patients with chronic respiratory disease.2,4 Strength training in PR is generally carried out using weight lifting exercises for the upper and lower limbs using gym equipment with heavy loads, at 70%–85% of the maximum weight that can be moved in a single preliminary maneuver (or 1RM test) and few repetitions.34 A recommended prescription would be to perform 1–3 sets of 8–12 repetitions of these exercises, in 2–3 sessions per week.2,4,34
Strength training requires greater patient supervision and proper staff training to ensure compliance and prevent potential injuries.4 The use of dumbbells and elastic bands is recommended in the home, as they are easy to use.34
Other Training ModalitiesOther important alternative strategies for peripheral muscle training in PR programs are transcutaneous electrical stimulation and electromagnetic stimulation. These are useful in patients who have difficulty in carrying out regular training, since they require little patient cooperation. Recommendation is weak (2C).2–4
Respiratory Muscle TrainingAs with general muscle training, the strength and resistance of respiratory muscles can be increased with certain forms of exercise such as interval training specifically targeting the breathing muscles.2–4,34 In patients with COPD, inspiratory muscle training (IMT) has been shown to improve muscle strength and resistance, helping improve dyspnea, functional capacity and quality of life.2–4,34 Despite this, and given the available evidence, adding IMT to general training within a PR program would be recommended if there is shown to be inspiratory muscle weakness (MIP<60cm H2O)2–4,35 (1B), so this type of training is not currently considered a fundamental part of the PR program (1B), although it could reasonably be indicated in other chronic respiratory diseases with respiratory muscle dysfunction. However, results so far are inconclusive and recommendation is weak34,36 (2C). In these patients, respiratory muscle training (RMT) should be avoided if there is hypercapnia, FVC <25% or rapid disease progression.34
RMT should generally be done twice daily, at an intensity of at least 30% of the MIP/MEP in sessions of around 15min.2–4,34 This type of training uses small, affordable devices that are easy to manage and allow the workload to be controlled. The most widely used are the Threshold® and Inspir® respiratory muscle trainers. The expiratory muscles can also be trained using abdominal exercises. In order to perform RMT properly, the patient should be instructed by specialized personnel, and if possible should learn to control their breathing pattern (expert recommendation).
EducationEducation is one of the main components of PR programs, despite the fact that it is difficult to quantify its direct impact on the benefits of PR programs.2–4
The basic aim is for patients and caregivers to understand and accept the disease and become involved in its management, becoming increasingly self-sufficient in terms of self-care and self-management.
- •
The term self-care is used in educational programs in relation to teaching the skills and abilities necessary for correct therapeutic compliance, bringing about a change in health behavior, and giving patients the emotional support they need to control their disease and live with the greatest possible functional autonomy.37
- •
Self-management, on the other hand, focuses on pharmacological therapy, teaching patients and their caregivers how to manage their drugs on a routine basis and when warning signs appear.38
Education is a continuous process that begins at diagnosis; it is shared responsibility involving patients, their caregivers and healthcare professionals (doctors, nurses, physiotherapists, etc.). Educational interventions should be adapted to each individual and agreed between the patient and professionals, so that together they can define treatment targets and design a strategy to achieve these goals.2
It is important for PR staff to know and understand the physiopathology and appropriate therapeutic interventions for each of the different diseases that may require PR.
Education programs included in PR are fundamentally designed for patients with COPD7 and asthma,39 although by extension they can be applied to other chronic respiratory diseases. The content is common to all groups, but is adapted according to the teaching resources available, and in particular to the circumstances and needs of each patient.
In general, such educational programs should be designed to give patients the following knowledge and skills2,40:
- -
Basic anatomy and physiology of the lung and respiratory system.
- -
Characteristics of the disease and symptom management.
- -
Healthy habits (diet, exercise, activities, vaccinations, etc.).
- -
Risk factors, such as exposure to smoke or other environmental pollutants.
- -
The medical treatment required at each stage of the disease (inhaled therapy, antibiotics, oxygen, ventilation, etc.), its benefits and side effects, and the strategies needed to strengthen and maintain adherence.
- -
Warning symptoms, to be able to prevent and treat exacerbations promptly, giving each patient individualized written instructions.
- -
Knowledge of energy saving strategies.
- -
Treatment of possible comorbidities.
- -
Knowledge of community resources and means of contacting the caregiver.
- -
Care and counseling in end-of-life decision-making.
Educational programs for respiratory patients have shown benefits in terms of improvement in health status and a reduction in the use of healthcare services.2,7,37,38,40 Specifically, the self-management strategy could be particularly beneficial for patients with poorer health status and/or high frequency of exacerbations.2 There has been wide debate on the possible impact of self-management on the overuse of drugs. However, this was not demonstrated in a Cochrane review,7 so this strategy may be safely recommended in patients with COPD.
In summary, international guidelines2–4 consider education to be an indisputable part of PR programs for COPD patients. Educational programs should include information on the disease and teach self-care and self-management strategies, with a high level of recommendation and moderate grade of evidence (1B).
Although the level of evidence for the benefits obtained by education in diseases other than COPD, such as bronchial asthma, is known, there is no specific recommendation within PR programs for these conditions, so it is considered, by extension, similar to that accepted for patients with COPD (1B).3
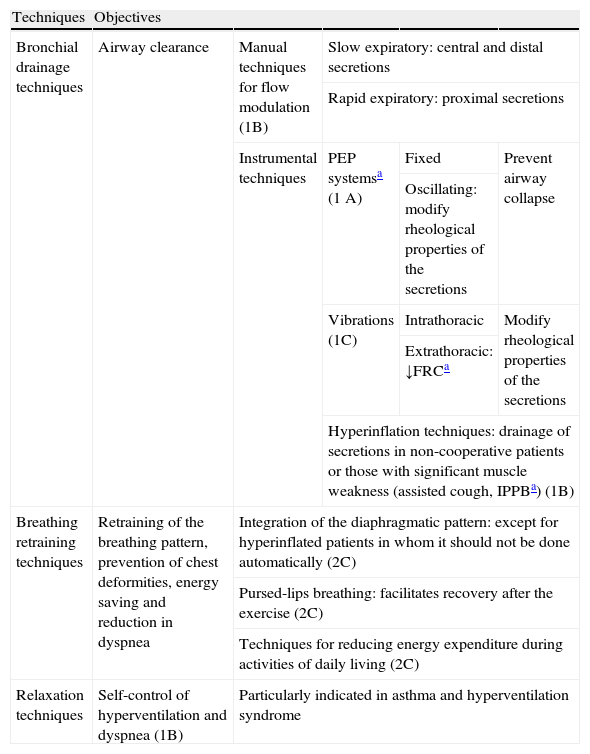
Chest PhysiotherapyChest physiotherapy (CPT) is also considered an important component of PR programs. This section will focus on bronchial drainage techniques, breathing retraining and relaxation techniques. Exercise training, oxygen therapy, mechanical ventilation and intervention in educational programs are also closely associated with the physiotherapist, although they will be addressed in other sections of the guidelines.
Bronchial Drainage TechniquesThe main aim of bronchial drainage techniques is airway clearance in patients with hypersecretion or difficulty expectorating. They can be divided into three groups: traditional CPT techniques, manual techniques based on flow modulation and instrumental techniques.
Traditional CPT techniques, such as postural drainage and manual percussion and vibration, are not recommended at present due to associated adverse effects, such as oxyhemoglobin (SpO2) saturation, onset of episodes of bronchospasm, increase in gastroesophageal reflux, and risk of rib injuries (1B).41
Manual techniques based on flow modulation (1B) can in turn be divided into slow expiratory techniques, used to drain central and distal airway secretions (Slow Expiration with Glottis Opened in Lateral Posture [ELTGOL], Autogenic Drainage [AD]), and rapid expiratory techniques for proximal secretions (Active Cycle of Breathing Technique [ACBT], Forced Expiration Technique [FET], cough).42
Instrumental techniques are aids to manual techniques and may be classified into three types: Positive Expiratory Pressure (PEP) systems, instrumental vibrations and hyperinflation maneuvers.
- -
PEP systems prevent airway collapse and reduce asynchronous breathing; in the case of an oscillating PEP, they also modify the rheological properties of the secretions (1A).43
- -
Extrathoracic instrumental vibrations help reduce secretion viscoelasticity and the functional residual capacity (FRC), while intrathoracic vibrations (interpulmonary percussive ventilation [IPV]) have similar effects as oscillating PEP (1C).43
- -
Hyperinflation maneuvers are very useful for draining secretions in non-cooperative patients or those with significant muscle weakness (assisted cough, intermittent positive pressure breathing [IPPB]) (1B).42
At present, there is no evidence to suggest the superiority of one technique over another, so the technique that best suits the patient (autonomy, adherence, preference, etc.) should be chosen.42
If inhaled antibiotic medication has been prescribed, these should be taken in the following chronological order during the session: inhalation of the bronchodilator, inhalation of mucolytic and/or hyperosmolar agents (1B), drainage of secretions and, finally, a dose of inhaled antibiotic.42,44
Breathing Retraining TechniquesBreathing retraining techniques are intended to retrain the breathing pattern, prevent chest deformation, promote energy saving and reduce the sensation of dyspnea. Despite the benefits of including diaphragmatic breathing, in the case of patients with hyperinflation this type of respiratory work can increase the sensation of dyspnea, overload the inspiratory muscles and reduce the mechanical efficiency of breathing (2C).42,45 Pursed-lips breathing facilitates recovery in patients with chronic obstructive disease and exercise-induced hyperinflation,2 although there is little evidence in this respect (2C).42
Relaxation TechniquesRelaxation techniques allow patients to control the hyperventilation and dyspnea caused by the anxiety generated by the disease itself. These interventions are particularly indicated in asthma and hyperventilation syndrome (1B).42
Table 1 shows an outline of the various chest physiotherapy techniques discussed in this section.
Chest Physiotherapy.
| Techniques | Objectives | ||||
| Bronchial drainage techniques | Airway clearance | Manual techniques for flow modulation (1B) | Slow expiratory: central and distal secretions | ||
| Rapid expiratory: proximal secretions | |||||
| Instrumental techniques | PEP systemsa (1 A) | Fixed | Prevent airway collapse | ||
| Oscillating: modify rheological properties of the secretions | |||||
| Vibrations (1C) | Intrathoracic | Modify rheological properties of the secretions | |||
| Extrathoracic: ↓FRCa | |||||
| Hyperinflation techniques: drainage of secretions in non-cooperative patients or those with significant muscle weakness (assisted cough, IPPBa) (1B) | |||||
| Breathing retraining techniques | Retraining of the breathing pattern, prevention of chest deformities, energy saving and reduction in dyspnea | Integration of the diaphragmatic pattern: except for hyperinflated patients in whom it should not be done automatically (2C) | |||
| Pursed-lips breathing: facilitates recovery after the exercise (2C) | |||||
| Techniques for reducing energy expenditure during activities of daily living (2C) | |||||
| Relaxation techniques | Self-control of hyperventilation and dyspnea (1B) | Particularly indicated in asthma and hyperventilation syndrome | |||
Patients with COPD have a high incidence of depression and anxiety.2–4,46 The lack of autonomy and the degree of disability resulting from their clinical condition aggravates these symptoms. These psychosocial disorders can cause cognitive changes, alteration in HRQoL and in the ability to carry out activities of daily living, as well as dependence on healthcare services. Anxiety can also cause changes in the breathing pattern and increase dynamic hyperinflation, resulting in increased dyspnea.
The main aim of psychosocial treatment is to get the individual to come to terms with their disease, gain as much independence and self-esteem as possible given their limitations, and to have good support from those around them. The most widely used psychosocial interventions are: (1) relaxation techniques, symptom control techniques or educational strategies aimed at changing lifestyle habits and acquiring skills in the control of dyspnea, panic or other attacks; (2) individual or group psychological support and advice; (3) creation of patient associations and (4) pharmacological treatment when necessary.2,3
Psychosocial aid has a disputed role in PR programs and the results are controversial.2–4 Currently available data indicate that this strategy can facilitate changes in lifestyle and symptom management, mainly dyspnea, by improving the breathing pattern through physiotherapy and education strategies within a multidimensional PR program3,4 (2C). Some randomized controlled studies have shown that PR reduces the symptoms of anxiety and depression,47,48 and improves the manner in which patients approach their disease,47 either without any specific therapy or including techniques such as psychotherapy.49,50 This improvement is more evident when the patient has a greater degree of anxiety or depression before beginning the PR program.51
One aspect that should not be neglected, due to its great emotional impact, is sexuality. In addition to individual or group therapy, teaching strategies on lower energy expenditure that facilitate sexual activity and personalized advice on the use of drugs or oxygen therapy are useful.2,52
In summary, to date there is some scientific evidence to suggest that psychosocial intervention is effective as treatment in patients with COPD, especially if it forms part of a multidimensional PR program (2C).3 Therefore, recent BTS guidelines specify that patients with depression or anxiety may benefit from PR programs that include psychosocial support.4
Nutritional SupportAlterations in the body composition of patients with chronic respiratory diseases are a systemic indication of severity, with most scientific evidence being available for COPD.53 Schols et al.54 showed that loss of fat-free mass is an independent predictor of mortality in COPD patients. In these patients, low body weight has also been associated with deteriorated lung function, reduced diaphragmatic muscle mass and lower exercise capacity.55 Considering the importance of body composition in COPD, international guidelines2–4 recommend the inclusion of nutritional support in PR programs.
The need to identify and treat changes in body composition in these patients is justified by high prevalence and association with morbidity and mortality, high energy requirements during exercise training that may worsen these abnormalities, and the greater potential benefit that could be derived from a structured training program combined with nutritional support.56,57
The best method for making a nutritional diagnosis of patients admitted to a PR program is the body mass index (BMI).58 Multidimensional indices such as the BODE take BMI into account, with a poorer prognosis in patients with values <21.59 Determination of the fat-free mass (FFM) or lean body mass, estimated by measuring skinfold thickness, is another useful parameter that can provide more precise information on body cell mass.60
Nutritional SupplementsIn recent years, various studies have been published in which different nutritional interventions have been shown to benefit body composition, exercise tolerance and HRQoL in patients with COPD included in PR programs.55,56,61,62 The main aim of these nutritional supplements is to enable patients to maintain their body weight and lean body mass within acceptable limits.
High energy foods (calories and proteins) enriched with macro- and micro-nutrients are used, in which essential amino acids (EAA) play an important role. Their beneficial effects on body weight and FFM have been demonstrated by Baldi et al.62 in a study that also showed the potential of EAA in the regulation of insulin-mediated signaling in protein and glucose metabolism. Weekes et al.,61 in a group of patients with COPD, demonstrated that nutritional support improved weight gain and HRQoL. Creutzberg et al.63 studied the effects of nutritional supplements administered for 8 weeks in malnourished COPD patients undergoing a PR program, and found an increase in lean body mass, muscle strength, exercise performance and quality of life. Another recent study has shown that the use of polyunsaturated fatty acids during an exercise training program reduces the levels of various systemic markers of inflammation, such as C-reactive protein, TNF-alfa and IL-8.64 These data justify the use of EAA as a valuable complement to physical exercises in PR programs aimed at stabilizing or even reversing the negative effects of lean body mass loss in these patients. In patients with COPD included in PR programs, creatine supplements do not improve exercise capacity, muscle strength or HRQoL, so their use is not recommended.65
Pharmacological InterventionsSome clinical trials have investigated the benefits of using growth hormone and anabolic steroids such as nandrolone, megestrol acetate and testosterone, with mixed results.66,67 The study by Pison et al.,68 carried out in patients with severe COPD and respiratory failure, showed the beneficial effects of testosterone within a PR program in improving body weight, FFM, exercise tolerance and patient survival. Despite these isolated, although promising results, we cannot recommend the routine use of anabolic supplements within PR programs.
ObesityMeal planning with nutritional education, calorie restriction, promotion of weight loss and psychological support is recommended in overweight and obese patients.58 Even though a target has yet to be established in relation to the magnitude of weight loss obtained after PR, comprehensive rehabilitation of obese patients may lead to weight loss and improvement in functional status and HRQoL.
In summary, a nutritional diagnosis should be established based on the body mass index (BMI) and the patient's nutritional risk, followed by a personalized eating plan based on proper food education, taking into account comorbidities and socio-economic and cultural factors. Available evidence suggests the use of nutritional supplements within a multicomponent PR program, with precise evaluation of results centered on changes in body composition, exercise tolerance and HRQoL.2 The data available does not recommend the routine use of anabolic supplements within PR programs. Nutritional interventions should last at least 12 weeks or while the patient remains in the PR program (2C). In diseases other than COPD, the guidelines suggest using common sense and including measures to combat malnutrition or excess weight, extrapolating the COPD results.2,4
Role of Oxygen Therapy and Ventilation in Pulmonary RehabilitationA distinction must be made between the immediate effects of oxygen while an exercise is being performed and its use as a component of training.
Oxygen supplementation during exercise, especially in patients with hypoxemia, increases the exercise capacity, decreases breathing requirements, reduces the respiratory rate and dynamic hyperinflation, and improves dyspnea and HRQoL,69 although these positive effects have not always been found.70
Supplemental oxygen therapy during exercise training should be assessed in two situations: patients with resting or exercise-induced hypoxemia, and patients who do not have hypoxemia. Patients who receive long-term continuous oxygen therapy should use it during training, generally increasing the oxygen flow prescribed at rest.
- -
Patients with hypoxemia: For safety reasons, the administration of supplementary oxygen during training for patients with resting or exercise-induced hypoxemia (1C) is justified, and SpO2 during training should be maintained above 90%. However, although the use of oxygen therapy greatly improves exercise performance, its effect on training parameters is inconsistent.3,4,71,72
- -
Patients without hypoxemia: The administration of supplementary oxygen during high intensity training programs in patients without exercise-induced hypoxemia can improve endurance (2C), although at present it remains unclear whether this in turn improves clinical results.73
The use of heliox or helium-hyperoxia, although it increases the inspiratory capacity, reduces dynamic hyperinflation and dyspnea and increases resistance time. However, it has not been shown to have sustained benefits, and is difficult and expensive to administer.74,75
Non-invasive positive pressure mechanical ventilation (NIV) reduces inspiratory muscle work, improves oxygenation of the quadriceps, reduces dyspnea and increases exercise capacity in some patients with COPD.76 Nocturnal home NIV combined with pulmonary rehabilitation in patients with severe obstruction and hypercapnic respiratory failure can optimize the benefits of PR in terms of exercise capacity,77,78 quality of life77–79 and gas exchange,79 probably due to the respiratory muscles having rested overnight.
Studies have also been conducted in which NIV was use to optimize exercise training, with mixed results. In one clinical trial in patients with COPD and moderate-severe obstruction (mean FEV1 44% of theoretical) in which this strategy was applied, no significant differences in dyspnea, leg fatigue, exercise tolerance or HRQoL were found between groups trained with spontaneous breathing or with ventilatory assistance.80 However, in another clinical trial in patients with more severe obstruction (mean FEV1 27% of theoretical), in the group receiving ventilatory assistance training intensity was 15.2% higher [P=.016 (95% CI, 3.2–27.1)], peak workload was 18.4% higher [P=.005 (95% CI, 6.4–30.5)] lactate level was lower [P=.09 (95% CI, 3.3–40)].81
In short, NIV as a complement to training in selected patients with COPD and severe obstruction produces modest improvements in exercise performance (2B).3
Pulmonary Rehabilitation Results in Patients With Chronic Obstructive Pulmonary DiseasePR is a fundamental part of treatment of patients with COPD and is recognized as such in most guidelines.2–4,82 PR programs should be compulsory for all patients with COPD who continue to be limited by symptoms despite following correct pharmacological treatment (1A).3,4
The objectives of PR in COPD are to improve symptoms and exercise capacity, reduce healthcare costs, and stabilize or reverse the systemic manifestations of the disease.3,4
Dyspnea is the most disabling symptom with the greatest impact on HRQoL in patients with COPD. PR has been shown to decrease dyspnea and improve exercise capacity and HRQoL in these patients (1A).3 It has also been observed that after a PR program, psychoemotional aspects such as anxiety and depression are improved (2B).3
These benefits of PR have generally been observed in patients with moderate obstruction, although improvements have also been demonstrated in patients with more severe disease (2C).83
Moreover, PR has been shown to reduce the number of days of hospitalization and use of healthcare services in patients with COPD (2B),3 so it is considered to be a cost-effective intervention with evidence level 2C.3 However, there is insufficient evidence to determine whether PR improves survival in these patients.3 It has only been possible to demonstrate that in patients who have taken part in a PR program after an exacerbation, the rate of subsequent hospital admissions and mortality have been reduced (1B).5
The benefits of PR do not appear to be linked with the location of the programs. Thus, home PR programs have shown improvements in dyspnea, exercise capacity and HRQoL similar to those obtained in hospital programs (1A).22–26,84
The issue of when PR programs should start is still under debate. Most guidelines suggest starting in a stable phase of the disease, however, recent studies have shown that initiating a program immediately after an exacerbation, in addition to being safe, is equally beneficial in terms of improving symptoms, exercise capacity and HRQoL, and reducing the number of hospital admissions (1B).2,4,5,85
Long-term BenefitsPR programs that include 6–12 weeks of training have been shown to improve exercise tolerance and HRQoL, and to reduce dyspnea and the number of hospital admissions in patients with COPD.2–4 However, the benefits obtained are gradually lost over 12–18 months (1A).3,86
Various factors can influence the long-term benefits of PR, including: the evolution of the disease itself,87 existence of comorbidities,88 the intensity, duration89 (2C) and location of the programs,22 and particularly, the use of maintenance techniques.90–96
So far, maintenance programs have demonstrated little efficacy in sustaining the benefits obtained from an intensive PR program, although admittedly few studies have been conducted in this respect (2C).2,3
All maintenance programs must take into account strategies such as self-management, defined as a program designed to support and help patients acquire the skills necessary to carry out specific medical regimes and to suggest behavioral changes that can improve disease control. However, few studies have evaluated self-management in COPD patients in relation to sustained activity or physical exercise (2C).38
There is increasing interest in designing models that sustain improvements gained in exercise capacity and HRQoL after the intensive phase of a PR program. It is unclear which maintenance programs would be most effective. In recent years, various controlled studies have been published that have included strategies for enhancing adherence to PR treatment, such as telephone monitoring, exercise monitors and cell phone applications with exercise speed controlled by a preset tempo.90,91
The frequency of interventions to maintain the benefits of exercise has been variable, with protocols that include supervising the exercise once a week, 3 times a week or even once a month. Some of these programs, mainly when supervised 3 times per week, could be considered to be a continuation of an intensive PR program, which may not be feasible in many healthcare systems. Studies that have evaluated monthly supervision have shown that benefits are generally lost, and exercise capacity diminishes at 12 months, indicating that this frequency of supervised training is insufficient to maintain any improvements. Two studies have shown that exercises supervised once a week maintained improvements in exercise capacity and HRQoL for periods greater than 12 months, although one of these studies used an initial 6-month pulmonary rehabilitation program, which is much longer than those commonly available,92 and the other was a randomized trial conducted in the intensive but not in the maintenance phase.93
Other designs, such as repeating programs every 2 years, have not been shown to be more effective: a significant number of patients are lost, and they are difficult to apply in clinical practice.2,94
Home rehabilitation without direct patient supervision can require fewer resources and include more patients.95 The strategy to follow will depend on the setting, and alternatives such as municipal facilities, incentive programs or exercise plans can be used (2C).3 Few studies have systematically evaluated the risk of adverse events with home rehabilitation, and if these occur, they are more related with exacerbations of the disease than with the PR intervention itself.95
Although no randomized studies have been conducted, telemedicine has recently begun to be used as means of monitoring and controlling compliance in PR maintenance programs, under the hypothesis that it is potentially useful for maintaining benefits in the long term, and can include a large number of patients.2,96
BTS guidelines4 recommend encouraging all patients to continue exercising after a PR program (1A), and good clinical practice recommends giving patients the opportunity to continue to engage in physical activity.
Pulmonary Rehabilitation in Chronic Respiratory Diseases Other Than Chronic Obstructive Pulmonary Disease and Other Diseases With Respiratory ComplicationsRespiratory symptoms such as dyspnea, as well as changes in exercise capacity or HRQoL, occur in almost all chronic respiratory diseases. Skeletal muscle function deteriorates in cystic fibrosis, bronchial asthma, obstructive sleep apnea-hypopnea syndrome (OSAHS) and lung cancer.97,98 In idiopathic pulmonary arterial hypertension, exercise intolerance is one of the most common symptoms and is related with respiratory and peripheral muscle dysfunction.99
There are no specific programs for each disease, and established programs for COPD patients should be adapted to each disease group.2–4
Bronchial AsthmaPhysiotherapy techniques should be included in PR programs, essentially to control attacks, and should include breathing retraining and relaxation techniques2,4,100 (1A). Aerobic training (cycling, swimming, treadmill) for at least 20min a day, twice weekly for at least 4 weeks, is a well tolerated therapy with no side effects that has been shown to increase oxygen consumption in patients with well controlled asthma (O2 consumption: 5.57mL/kg/min; 95%CI [4.36–6.78]) (1A).101 Patients who develop exercise-induced asthma should use fast-acting β-2 agonists before starting training, as well as doing a preliminary gradual warm-up (2B).101
Cystic Fibrosis and BronchiectasisChest physiotherapy, and specifically secretion drainage techniques, either manual or instrumental, is essential in these diseases (1A).2,4,42,102 The physiotherapy techniques should be chosen according to the patient's preference, as none has been shown to be more effective. Self-administered techniques are generally recommended to facilitate compliance. Physiotherapy should be carried out once to three times daily, after bronchodilator treatment and before inhaled antibiotics, if prescribed (1B). Although it is unclear whether patients with non-productive cough would also benefit from physiotherapy techniques, experts generally agree that they should at least undergo physiotherapy during exacerbations (2C).102 Exercise, meanwhile, should be moderate to intense for at least 30min a day, 3–4 times a week, or failing that, daily moderate physical activity should be recommended (1B).2,4,42 One aspect mentioned in other international guidelines involves control of infections: a distance of at least 1m should be maintained between cystic fibrosis patients, given the potential risk of cross-infections with resistant microorganisms.2
Idiopathic Pulmonary Arterial HypertensionRecent studies have shown an improvement in exercise capacity and HRQoL after aerobic training, together with upper limb training, in sessions spread over 3–7 days per week, for 7–15 weeks, with no adverse effects having been recorded.2,4,103 Likewise, patients with chronic thromboembolic pulmonary hypertension who do aerobic training together with low intensity strength training show an improvement in HRQoL and exercise capacity, with no major adverse events.104 However, although the results are promising, further studies are required to establish the safety of PR (2C).2,3
Interstitial Lung DiseasesThese are characterized by dyspnea and hypoxemia that worsen with exercise. These symptoms are due, among other causes, to: lack of pulmonary distention, impaired gas exchange, altered breathing pattern (superficial respiration and dynamic hypoinflation) and systemic corticosteroid treatment.105 As a result, patients with interstitial lung diseases (ILD) tend to be sedentary, with functional limitation and worse HRQoL.106 Guidelines usually recommend limiting physical exercise in patients with ILD. However, at present, evidence suggests that PR may be an effective and safe treatment.107,108 Thus, when training is indicated individually, with an intensity established by a cardiopulmonary exercise test and taking into account possible associated comorbidities such as pulmonary arterial hypertension, cardiac impairment or arrhythmias, major benefits can be obtained in terms of symptoms and exercise capacity. Although the most appropriate exercise protocols are not yet well established, symptom-limited low intensity aerobic training is generally recommended (2B).3,109
Obstructive Sleep Apnea-Hypopnea SyndromeRandomized controlled studies with a limited number of subjects have been conducted that show that 12 weeks of moderate intensity aerobic training 4 days a week, together with 2 days a week of resistance training, reduces the sleep apnea-hypopnea index and objectively and subjectively improves the quality of sleep,110 depressive symptoms and drowsiness, in addition to other benefits (2C).111
Lung CancerPatients diagnosed with lung cancer usually undergo major changes in HRQoL, mainly due to impairment of their physical condition (weakness, anorexia, cachexia), emotional changes secondary to their disease, and to treatments such as chemotherapy or radiotherapy. If a previous chronic disease (basically COPD) is also present, dyspnea usually worsens the condition.
It is reasonable to consider that a PR program could result in an improvement in these patients. Few studies have shown that respiratory physiotherapy techniques improve symptoms in this patient group with112; exercise training is clearly much more effective with respect to improving not only symptoms by also exercise capacity and HRQoL.113–115
Neuromuscular DiseasesThe control of respiratory secretions is, together with the prevention of food aspiration and maintenance of adequate alveolar ventilation, fundamental for the management of respiratory problems in neuromuscular diseases (NMD) (1C) and is the basis of PR in these entities10,116–120 (see on-line section).
Pulmonary Rehabilitation and Thoracic SurgerySurgical treatment of lung cancer as well as new COPD therapies (lung volume reduction surgery and lung transplant) or other surgeries considered high risk in patients with chronic respiratory diseases, require optimal clinical status and, therefore, the multidisciplinary approach of PR is a critical component of the therapeutic strategy in these situations.121
Generally, in order to prevent post-operative complications in patients undergoing chest or upper abdominal surgery, above all with prior respiratory disease, CPT has a very important pre- and post-operative role. Worth mentioning are breathing retraining techniques including incentive spirometry (IS) and airway clearance maneuvers when there are secretions.122,123 Another technique to consider is continuous positive airway pressure (CPAP).121 However, no studies to date have shown any advantage of this treatment over CPT techniques. Intermittent positive pressure breathing (IPPB) may also be effective in preventing post-operative lung complications, but because of its higher cost and relatively high incidence of abdominal distention it is not a treatment of choice.124 Although CPT for patients who are candidates for thoracic surgery is strongly recommended, no randomized controlled studies have so far confirmed its effectiveness. Some non-randomized studies suggest that CPT may reduce the risk of atelectasia and hospitalization, but it does not seem to affect the incidence of pneumonia or morbidity.123 Therefore, the role of CPT in thoracic surgery has a low grade of evidence but a high recommendation (1C).
Exercise training is one treatment strategy to consider in patients undergoing thoracic surgery. In patients with chronic lung disease who undergo tumor resection, the best approach would be to instigate a training regime in addition to CPT in the weeks prior to surgery to improve muscle strength and resistance, and to restart these strategies as soon as possible post-operatively.
In patients who are candidates for lung volume reduction surgery or lung transplant, the full PR program, including exercise training, should be commenced as soon as possible.
PR in lung volume reduction surgery candidates is safe and effective.125 The NETT study showed that 20% of patients waiting for lung volume reduction surgery improved with PR to the point where the surgery was no longer required.126 Improvements affected lung function, gas exchange, exercise capacity and HRQoL.127
Patients that are candidates for lung transplant have greatly impaired exercise capacity; therefore, PR is an essential part of the therapeutic strategy. Prior to the transplant, the main objective of PR is to optimize and maintain the patient's functional status while closely monitoring the underlying disease. Moreover, PR may be a good starting point to better refine candidate selection, both through the assessments carried out (walk test) and monitoring program compliance, which may help to detect non-compliant patients.128 A recent review confirmed that PR is beneficial for lung transplant candidates in terms of muscle function, exercise capacity and bone mineral density.129 However, because few studies have been made, it is unclear whether improved exercise capacity prior to surgery can reduce post-operative complications and mortality.129 Post-transplant, PR is important since exercise capacity and myopathy is greatly impaired; training should be started gradually and early. In these patients, continuous and interval training have been shown to be equally effective,130 both in hospital or at home.131
At present, considering the studies conducted to date, it can be said that PR has a high level of evidence and recommendation for candidates for lung volume reduction surgery or transplant (1A).
Other Aspects of Pulmonary RehabilitationPhysical ActivityPhysical inactivity is common in patients with COPD.132 These patients adopt a more sedentary lifestyle, and it has been shown that the time that they remain active correlates poorly with the degree of airflow obstruction.133 Inactivity is a factor of poor prognosis and is associated with higher mortality.134,135 Therefore, one aim of PR programs is to increase patients’ physical activity. Studies conducted in this respect have been unable to reliably demonstrate that improvements in exercise tolerance in patients following a PR program implies a greater degree of physical activity in their daily life.136–138 Transferring improvement in exercise capacity achieved in PR programs to daily life is a future challenge that must involve changing the patient's behavior and habits.139
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Güell Rous MR, Díaz Lobato S, Rodríguez Trigo G, Morante Vélez F, San Miguel M, Cejudo P, et al. Rehabilitación respiratoria. Arch Bronconeumol. 2014;50:332–344.