To describe the epidemiology of tuberculosis and analyzing the differences among native and immigrant patients in Area III of the Region of Murcia.
MethodsCohort study of tuberculosis cases reported to the Epidemiological Surveillance Service from 2004 to 2009. Data collection was performed through the System of Notification Diseases, reviewing clinical files and epidemiological surveys.
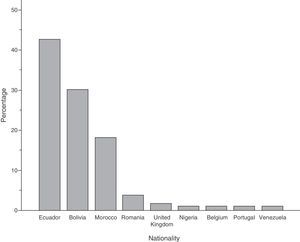
ResultsOne hundred and sixty two cases were detected; 110 (67.9%) were immigrants, whose incidence rates ranged from 43.4 to 101.2 cases per 100000 inhabitants. Ecuador (42.7%), Bolivia (30%) and Morocco (18.2%) were the main nationalities.
Immigrants were younger than Spanish population (P<.001). The overall diagnostic delay was 50.5days: 59.5 in Spanish and 47 in foreigners. Moroccans had higher proportions of extrapulmonary TB (P=.02). Mainly, immigrant population took treatment with four drugs (P<.001). Natives had better treatment adherence (P=.04). Spanish cases of tuberculosis were associated with smoking (P<.001), the same as alcohol consumption (P=.01) and injection drug use (P<.001), nevertheless in the foreign-born population the most relevant risk factor was overcrowding (P<.001).
ConclusionsThe incidence of tuberculosis rates are higher among immigrant population, where the main risk factor is overcrowding. In contrast, Spanish cases are associated with toxic substances consumption and increasing age.
Describir las características epidemiológicas de la tuberculosis y analizar las diferencias existentes entre pacientes autóctonos e inmigrantes en el ÁreaIII de Salud de la Región de Murcia.
MétodosEstudio de cohortes retrospectivo de casos de tuberculosis declarados al Servicio de Vigilancia Epidemiológica en el periodo 2004-2009. La recogida de datos se realizó a través del sistema de enfermedades de declaración obligatoria, la revisión de historias clínicas y las encuestas epidemiológicas.
ResultadosSe detectaron 162casos, y 110 (67,9%) correspondían a inmigrantes, cuyas tasas de incidencia oscilaron de 43,4 a 101,2 casos/100.000 habitantes. Los principales países de procedencia fueron Ecuador (42,7%), Bolivia (30%) y Marruecos (18,2%).
En el momento del diagnóstico, el colectivo inmigrante era más joven que la población española (p<0,001). El retraso diagnóstico global fue de 50,5días: 59,5 en españoles y 47 en extranjeros. Los marroquíes presentaron mayor proporción de tuberculosis extrapulmonares (p=0,02). La población inmigrante realizó mayoritariamente tratamiento con 4fármacos (p<0,001). La población autóctona tuvo mejor adherencia al tratamiento tuberculostático (p=0,04) y la enfermedad se asoció al tabaquismo (p<0,001), al alcoholismo (p=0,01) y al uso de drogas parenterales (p<0,001), mientras que en el colectivo inmigrante el factor de riesgo más relevante fue el hacinamiento (p<0,001).
ConclusionesLas tasas de incidencia de tuberculosis son muy elevadas en población inmigrante. El principal factor de riesgo medible en este colectivo es el hacinamiento, mientras que en la población española se asocia al consumo de sustancias tóxicas y a una mayor edad.
The problem of immigration and tuberculosis (TB) is an aspect of the phenomenon of globalization.1 Shifting demographics, in addition to encouraging social and economic dynamism, also facilitates the introduction of diseases into regions where they were relatively rare or well controlled. TB is considered a health problem of the first order on a worldwide level,2 and its prevalence is clearly affected by inequalities in healthcare originating from economic, political and social inequalities within the community.3 Economic migrants, being generally from social classes with fewer resources, are in themselves a risk group for TB. Given their vulnerable position, attention needs to be paid to these groups to detect their healthcare needs and demands. Many industrialized countries have experienced changes in the epidemiological pattern of TB, with evidence of 2 trend curves: the descending curve, reflecting the trend of the disease in the native population, and the ascending curve, representing the immigrant population, particularly those from counties with a high prevalence of tuberculosis.4,5
In Spain, as in other European countries,6 the increase of migration-related TB is detected to a greater or lesser extent in all autonomous communities, with higher rates being observed in areas with a greater concentration of immigrants.7
This has been the general scenario for the last few years, and there is little doubt that the conditions in which these immigrants live in the host country produce a series of difficulties, including access to health services, the language barrier, geographic mobility, an irregular legal situation, difficulties in locating subjects and providing care, poor therapeutic compliance and treatment funding. All these factors add up to serious problems in the diagnosis, treatment and cure of these patients, and in the contact tracing of family members and cohabitants, thus facilitating the spread of the infection and the development of new cases.8
The region of Murcia is an example of the foregoing pattern, and despite the influence of the recent migratory phenomenon, few papers have been published along this line of investigation, suggesting the need to analyze the epidemiological profile of the TB patient in this community and determine the effect of immigration in this setting.
MethodsThis was a retrospective cohort study that included all cases of TB among the residents of Healthcare Area III reported to the Epidemiological Surveillance Service of the Region of Murcia between January 1, 2004 and December 31, 2009. The exposed cohort consisted of subjects who were immigrants, and the non-exposed cohort was the group of Spanish subjects. Healthcare Area III is a semiurban zone that is one of the 9 healthcare areas of the Region of Murcia. It has a total of 170663 inhabitants and 19% are immigrants, according to the data from the National Institute of Statistics (INE) for 2009.
For the definition of TB, the criteria approved by the National Epidemiological Surveillance Network9 in 2003 and the definition proposed by the European Centre for Disease Prevention and Control10 in 2007 were used. Pulmonary TB was that affecting the lung parenchyma and the tracheobronchial tree. For TB with multiple organ involvement, the pulmonary site was always considered principal and the rest additional.
The TB epidemiological survey produced by the Epidemiological Surveillance Service and the TB Prevention and Control Program was used for data collection. The surveys were always administered by one of the 2 program nurses and were completed with hospital and primary care medical records. Sociodemographic variables (age, sex, country of origin, time of residence in Spain) and clinical variables associated with tuberculosis (site, symptoms, time to diagnosis, clinical laboratory data, number of drugs used, therapeutic compliance, final outcome) were analyzed. In addition, risk factors, such as disease history, alcoholism, smoking habit, use of intravenous drugs and overcrowded living conditions (ratio of more than 2.5 persons per sleeping quarter) were examined. The source of the report was analyzed to evaluate the representativeness and completeness of the epidemiological surveillance system.
Data were analyzed using the SPSS software package (version 19.0 for Windows). Qualitative variables were expressed as frequencies and percentages. For symmetric variables, the mean with 95% confidence interval (CI) and standard deviation (SD) were calculated, and the median and interquartile range (P25, P75) for asymmetric variables. Proportions were compared with the chi-squared (χ2) test and absolute variables were compared using the Student's t-test and variance analysis. Odds ratios (OR) with their corresponding 95% CI were calculated to measure association. The analysis of factors associated with treatment discontinuation, fatality, or double localization were analyzed by logistical regression, with the exploratory inclusion of possibly influencing factors. The INE population estimates for 2004–2009 were used for calculating incidence rates. Trends in the rates were studied by linear regression and the goodness of fit was evaluated with the coefficient of determination r.2 For the analyses, statistical significance was set at 5% (P≤.05).
ResultsA total of 162 cases of TB were detected between 2004 and 2009. Of these, 63.6% (n=103) occurred in men. The overall mean age was 33.81 years (SD=16.08; 95% CI: 31.32–36.31). The main source of cases of TB was the registry of reportable diseases (85 cases [52.5%]). (Table 1).
Distribution of Tuberculosis (Tb) Cases by Year and Origin.
| Years | Total TB patients | TB Spanish patients (%) | TB non-Spanish patients (%) |
| 2004 | 31 | 11 (35.5) | 20 (64.5) |
| 2005 | 30 | 9 (30.0) | 21 (70.0) |
| 2006 | 24 | 10 (41.7) | 14 (58.3) |
| 2007 | 24 | 7 (29.2) | 17 (70.8) |
| 2008 | 29 | 5 (17.2) | 24 (82.8) |
| 2009 | 24 | 10 (41.7) | 14 (58.3) |
| Total | 162 | 52 (32.1) | 110 (67.9) |
The comparative analysis between the 2 study populations is described in Tables 2 and 3.
Patient Distribution by Variables Studied and Bivariate Analysis of Spanish vs Non-Spanish.
| Variables | Spanish (%) | Non-Spanish (%) | OR | 95% CI | P |
| Sex | |||||
| Male | 37 (71.2) | 66 (60) | 1.64 | 0.81–3.35 | .23 |
| Female | 15 (28.8) | 44 (40) | |||
| Age (years) | |||||
| Mean (SD) | 42.1 (20.97) | 29.9 (11.34) | <.001 | ||
| Age group | |||||
| <18 | 5 (9.6) | 9 (8.2) | 1.19 | 0.38–3.70 | .99 |
| 18–40 | 23 (44.2) | 85 (77.3) | 4.28 | 2.12–8.68 | <.001 |
| 40–64 | 15 (28.8) | 16 (14.5) | 2.38 | 1.06–5.26 | .05 |
| >64 | 9 (17.3) | 0 | 48.26 | 2.75–847.35 | <.001 |
| Site | |||||
| Pulmonary | 37 (71.2) | 85 (77.3) | 1.38 | 0.65–2.91 | .52 |
| Mixeda | 10 (19.2) | 6 (5.5) | 4.13 | 1.41–12.08 | .01 |
| Treatment | |||||
| 3 drugs | 25 (49) | 13 (11.9) | 7.10 | 3.20–15.77 | <.001 |
| 4 drugs | 26 (51) | 96 (88.1) | |||
| Resistance | |||||
| 1st line resistance | 2 (5.9) | 12 (13.6) | 2.53 | 0.53–11.90 | .38 |
| INH resistance | 2 (5.9) | 10 (11.4) | 1.38 | 0.27–7.01 | .69 |
| Treatment outcome | |||||
| Discontinued | 2 (4.3) | 10 (9.2) | 2.22 | 0.47–10.53 | .49 |
| Lost-to-follow-up due to move | 0 | 10 (9.2) | 10.86 | 0.62–189.21 | .09 |
| Compliance | 44 (95.7) | 89 (81.7) | 4.94 | 1.11–22.11 | .04 |
| Deatha | 6 (11.5) | 1 (0.9) | 5.15 | 0.46–58.82 | .18 |
| CCI | |||||
| All TB | 44 (84.3) | 96 (86.5) | 1.02 | 0.39–2.70 | .97 |
| BK+pulmonary TB | 22 (84.6) | 59 (90.6) | 1.89 | 0.45–6.67 | .59 |
| Delay in diagnosis | |||||
| Median (IQR) | 59 (19–113) | 47 (15–95) | .45 | ||
| IVDA | |||||
| >16 years | 6 (12.8) | 0 | 31.48 | 1.79–571.62 | <.001 |
| Smoking habitb | |||||
| >16 years | 27 (57.4) | 25 (25.0) | 4.05 | 1.94–8.44 | <.001 |
| Alcoholism | |||||
| >16 years | 10 (21.3) | 6 (6.0) | 4.23 | 1.44–12.48 | .01 |
| Overcrowding | |||||
| Home environment | 2 (3.8) | 34 (31.2) | 11.33 | 2.60–50 | <.001 |
IVDA: intravenous drug addict: BK: bacilloscopy; CCI: conventional contact investigation; CI: confidence interval; INH: isoniazide; IQR: interquartile range; OR: odds ratio; P: level of statistical significance.
Multivariate Analysis: Immigrants Versus Natives.
| Variables | OR | 95% CI | P |
| Age | |||
| <16 | 5.83 | 0.33–102.03 | .23 |
| 16–64 | 13.92 | 1.27–151.96 | .03 |
| >64 | 1 | ||
| Treatment | |||
| 4 drugs | 8.30 | 2.71–25.41 | <.001 |
| 3 drugs | 1 | ||
| Smoking habit | |||
| Non-smoker | 7.24 | 2.73–19.23 | <.001 |
| Smoker | 1 | ||
| Overcrowding | |||
| Yes | 19.73 | 2.24–174.17 | .007 |
| No | 1 | ||
CI: confidence interval; OR: odds ratio; P: level of statistical significance.
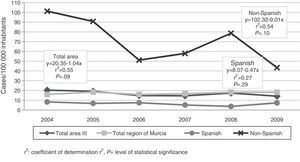
The immigrant group accounted for 67.9% (n=110) of all cases, and high incidence rates were reported in this group throughout the study period (Fig. 1), reaching 101 cases per 100000 inhabitants in 2004. The foreign population originated from 9 different countries, mainly Ecuador, Bolivia and Morocco (Fig. 2).
A total of 63.6% (n=70) of the cases had been living in Spain for less than 5 years when they were diagnosed with TB (mean 4.65 years [SD=3.71; 95% CI: 3.95–5.36]). There were no significant differences for gender; however there were differences among the main nationalities [F(2, 31,6)=5.71; P<.001]: patients from Bolivia had been living in Spain 2.63 years less than those from Ecuador (95% CI: 1.61–3.66; P<.001).
The most common site of disease was the lung, accounting for 75.3% (n=122) of the cases. The Moroccan population was determined to have the greatest proportion of extrapulmonary TB (OR=4.26; 95% CI: 1.40–12.99; P=.02).
With regard to the clinical picture, 90.7% (n=146) of the patients were symptomatic before diagnosis of TB, with cough being the symptom observed in most respiratory TBs (78.7%), at equal rates in both groups. When the radiological findings were analyzed, 36.9% (n=45) of pulmonary TBs showed cavitation. The median delay before diagnosis was 50.5 days (P25: 16.8; P75: 103.50) from the date of onset of symptoms until the date of diagnosis, and 53 days (P25: 20; P75: 110) in pulmonary TBs with positive bacilloscopy. No significant differences were observed in the type of site or in the main foreign nationalities: in this cohort, the association between years of residence in Spain and delay in diagnosis was ruled out. A total of 87.7% (n=142) of cases were hospitalized during the onset of their disease, and there was a positive association with pulmonary presentation (OR=4.76; 95% CI: 1.80–12.57; P=.002). In the laboratory, bacilloscopy was performed in 95.1% (n=154) of the patients. These were positive in 64.9% (n=100) and in 75% (n=91) of the pulmonary TBs. One hundred and forty-eight (148) cultures were made and 119 Mycobacterium tuberculosis, 1 M. bovis, 1 M. caprae and 1 M. africanum were isolated. Drug sensitivity testing was performed on all positive cultures, and 11.5% (n=14) were resistant to first line drugs. Resistance to isoniazide was observed in 9.8% (n=12) of the cases with positive culture. There were 3 cases of multi-resistant TB, all in the immigrant population.
Trends in the tuberculostatic treatment regimen varied over the years, from 52.9% of subjects receiving a 4-drug regimen in 2004 to 80.8% in 2009 (XTL2=17.58; P=.001). The rate of overall compliance (number of cures+number of completed treatments×100/number of cures+number of completed treatments + discontinuations+cases lost-to-follow-up) was 85.8% (n=133). There were 7 deaths during the study period, representing 4.3% of all cases. Five patients with pulmonary TB and 2 with meningeal TB died; the latter showed a significant case fatality rate (OR=61.2; 95% CI: 4.74–791.99; P<.001).
DiscussionIn this study, TB in the immigrant population was much more common than the 32% reported in Spain7 in 2012 but similar to the rates seen in other European countries with a longer tradition of migration,6 reflecting the growing rates of migration to this country in recent years. The incidence rates are also very much higher in this population group, although there is a clear downward trend, particularly since 2005, possibly related with the introduction of Royal Decree 2394/2004, approving the legalization of immigrants in Spain.11 High incidence rates among the foreign population were also reported by González-Moreno et al.,12 although in their series the trend was toward growth throughout the study.
Similar to other studies, it was found that the disease occurred more commonly during the subject's first 5 years of residence in Spain.13 A recent study carried out in the United Kingdom,14 where 75% of cases affect the immigrant population, suggests that strategies for the detection of latent tuberculous disease at the time of entry into the country should be introduced, particularly for specific risk groups, with the aim of preventing exogenous reinfection from endogenous reactivation of the disease that would normally occur within the first 5 years after arrival.
In this analysis, it was also confirmed that the Bolivian population became ill before subjects from Ecuador. This can be explained by the fact that migration of Ecuadorians to the Region of Murcia began later.
As in other national15 and international16 studies, immigrants in this study were younger than the native population, reflecting the typical young TB-infected immigrant who has left his or her country in search of better labor and economic opportunities: individuals over 65 years of age are rarely found among the group known as economic immigrants.
With regard to disease site, the figures are similar to those published in Spain,7 in which pulmonary TB is the main form of presentation. However, as regards geographical origin, extrapulmonary TB was most commonly found in Moroccan patients, as also reported by Te Beek et al.17 in a study conducted in Holland.
Delay in the diagnosis of tuberculosis, an important indicator of the effectiveness of TB prevention and control programs, was greater than the 30 days stipulated in the WHO guidelines,2 but similar to that found in the study of Altet et al.,18 in which difficulty in accessing the healthcare system, particularly among the immigrant population, is given as a causative factor. The greater delay in the native population suggests that there is a low diagnostic suspicion in this segment of the population, so feedback on this issue among healthcare professionals would be of interest.
TB can be treated in the patient's home. However, in this study there was a greater trend toward hospitalization, especially in pulmonary presentations, due to the impact that this form of the disease can have on the community. Although hospital admission is not justified, figures were higher than the 56% reported by García-Fernández et al.19 in Madrid. It is worth mentioning that this not only involves high healthcare costs, as explained in the study by Montes-Santiago et al.,20 but also increases the risk of nosocomial transmission.
The use of bacteriological diagnostic testing was quite acceptable and a higher number of pulmonary TB cases had positive bacilloscopy than reported in other Spanish studies.21,22 This finding is important from an epidemiological point of view, since it is these infective forms that spread the disease, and their early detection is a priority in TB control programs.
Resistance to isoniazide in this study was higher than the 5.3% found in the study performed in this healthcare area between 1999 and 2004.23 However, other authors have also described similar resistance rates.13 In line with the literatures,23,24 the highest rates of resistance were found in the immigrant population.
With regard to the initial tuberculostatic regimen, the immigration population was most often treated with the 4-drug regimen, since the study was performed during the years in which the ethambutol-free regimen was still being used for Spanish patients, and when resistance to isoniazide was less than 4%; at present, the majority of drug regimens in this healthcare area are 4-drug schedules, as suggested by the new treatment guidelines.25
Therapeutic compliance is of vital importance for eliminating endemic tuberculosis. On the basis of the above, completion of tuberculostatic treatment was slightly higher than in the study previously performed in this region.23 Unfortunately, the 94% rate reported in the ECUTTE26 study was not achieved, nor was the 90%–95% completion rate suggested as acceptable in developed countries.27 As reflected in other studies,23,26 the proportion of patients with satisfactory progress was greater in the Spanish population, and immigrant status was a risk factor for lack of compliance. It should be mentioned that the characteristics of some groups of immigrants are conducive to deficient treatment compliance; the main reasons mentioned by authors28 include the language barrier, cultural differences, an irregular legal or administrative situation, and high geographical mobility. This results in a vulnerable group that should be allocated the resources required to ensure correct control.
Almost half of the patients had some type of social risk, corroborating the idea of TB as the social disease par excellence. As reflected in the literature, the poorest and most economically disadvantaged groups, that include recent immigrants, are those most ravaged by this disease.29
Similar to García-García et al.,15 the authors found that in the native population the disease was associated with smoking and alcoholism, while in the immigrant group the most significant risk factor was overcrowded living conditions. This is a setting that is always associated with social and economic hardship, as confirmed by the findings of the geostatistical study of Gómez-Barroso et al.,30 in which overcrowding was identified as one of the predictive disease variables.
Although contact tracing was extensive in both study groups, when the completeness indicator was evaluated, it was seen to fall below the threshold of 90% for the TB population proposed in the guidelines,31 and the recommended 100% for bacilliferous pulmonary TB. Other studies32 provide similar and even far lower figures,33 suggesting that there is a significant percentage of cases in which contacts are not traced, despite the risk this entails, particularly in the case of bacilliferous pulmonary TB.
Case fatality rates in this series were higher than those published in the multicenter Spanish study.15 In line with the findings of González-Moreno et al.,12 there was a higher death rate among meningeal presentations.
TB incidence and morbidity are usually determined from mandatory reporting procedures, but there is evidence that this type of passive registry underestimates the number of disease cases.34 This was also observed in this study, since only a little over 50% of the cases had been reported via this system. This important fact is a limitation of our study, since only cases detected and/or reported to the program were analyzed, and the real number of cases of TB in this region may be underestimated.
A further limitation of the study is its retrospective character, however, the epidemiological survey is always administered directly to the patient by the same professionals, whether in the hospital or at home, or by telephone if direct contract is impossible, thereby ensuring homogeneity in data collection. The nursing staff is also responsible for checking both laboratory data and therapeutic compliance, the final outcome, and for tracing contacts.
When the epidemiological characteristics of TB have been determined, it would be interesting to evaluate if a greater cultural adjustment and/or longer time of residency in Spain produces variations in the patient profile; this route of investigation remains open.
The main conclusions from this study are that the incidence rates of TB and overcrowding are very high in the immigrant population. In the Spanish population, however, the disease is associated with the consumption of toxic substances and older age, suggesting that resources should be channeled in this direction.
Finally, the current climate of socioeconomic crisis may produce a marked increase in the incidence of this disease among the most disadvantaged groups. For this reason, political leadership and the different healthcare strata must combine efforts to put a stop to TB.
FundingThis study did not receive any type of grant or assistance.
AuthorshipAll of the authors contributed significantly to the study design, the writing and critical review of the manuscript, met the conditions for authorship and approved the final version for publication.
Conflict of InterestsThere is no conflict of interests associated with this article or its authors.
Our thanks to all those who make us feel that our daily work is worth the effort, without whom this noble profession would not be possible, our PATIENTS, who have passed in and out of our lives, teaching us things that are not found in the textbooks and never taught at university.
Please cite this article as: Molina-Salas Y, Lomas-Campos MM, Romera-Guirado FJ, Romera-Guirado MJ. Influencia del fenómeno migratorio sobre la tuberculosis en una zona semiurbana. Arch Bronconeumol. 2014;50:325–331.