The most common causes of non-malignant central airway obstruction are post-intubation and post-tracheostomytracheal stenosis, followed by the presence of foreign bodies, benign endobronchial tumors and tracheobronchomalacia. Other causes, such as infectious processes or systemic diseases, are less frequent. Despite the existence of numerous classification systems, a consensus has not been reached on the use of any one of them in particular. A better understanding of the pathophysiology of this entity has allowed us to improve diagnosis and treatment. For the correct diagnosis of nonspecific clinical symptoms, pulmonary function tests, radiological studies and, more importantly, bronchoscopy must be performed. Treatment must be multidisciplinary and tailored to each patient, and will require surgery or endoscopic intervention using thermoablative and mechanical techniques.
Las causas más frecuentes de patología obstructiva no maligna de la vía aérea central son las estenosis postintubación y postraqueotomía, seguidas por los cuerpos extraños y la traqueobroncomalacia. Otras causas, como las secundarias a procesos infecciosos y enfermedades sistémicas, son menos frecuentes. A pesar de la existencia de numerosas clasificaciones, todavía no se ha alcanzado consenso sobre la utilización de alguna de ellas en concreto. Un mejor conocimiento de su fisiopatología nos ha permitido aumentar el diagnóstico y mejorar su tratamiento; su presentación clínica inespecífica exige la realización de diversos estudios funcionales, radiológicos y fundamentalmente endoscópicos para su correcto diagnóstico. El tratamiento debe ser multidiciplinario e individualizado, requiriendo tratamiento quirúrgico o endoscópico mediante diferentes técnicas termoablativas y mecánicas.
Obstruction of the central airway, trachea and primary bronchi is a common problem in medical and surgical settings. The incidence of this disorder seems to be rising due to the epidemic of lung cancer; however, the growing number of benign obstructive pathologies also contributes to this trend, primarily due to the use of artificial airways.1 Multidisciplinary management and progress in the use of different radiological and endoscopic tools have led to an improvement in the diagnosis and treatment of these conditions.
The aim of this review is to examine the causes of benign central airway obstruction considered most important by the authors, including intubation, tracheotomy, tracheobronchomalacia (TBM), infectious processes (tuberculosis) and systemic diseases (sarcoidosis, amyloidosis, Wegener's granulomatosis, relapsing polychondritis, tracheobronchopathia osteochondroplastica), and finally, idiopathic tracheal stenosis and post-lung transplantation bronchial stenosis.
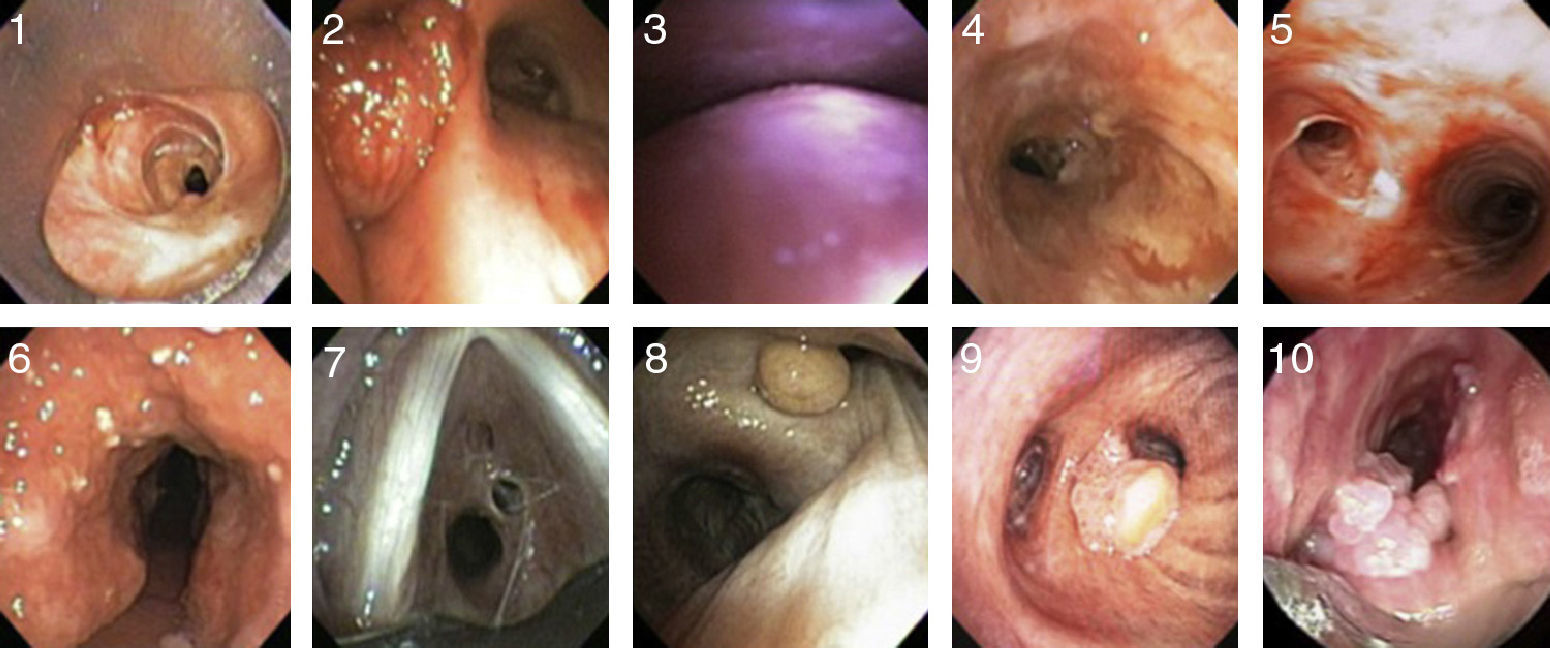
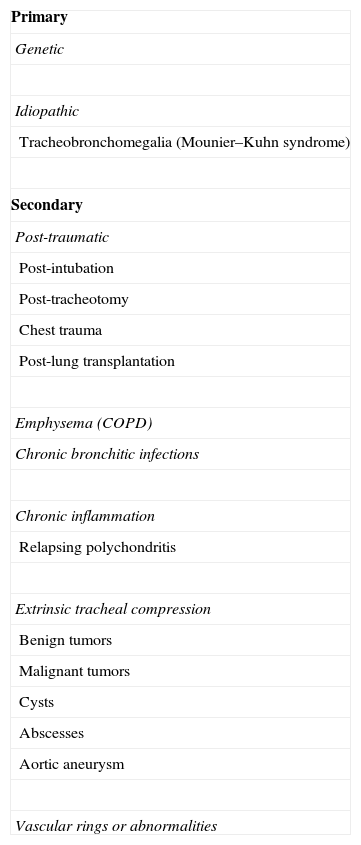
Etiology and ClassificationThere are many causes of central airway obstruction (Table 1), the most common being associated with orotracheal intubation and tracheotomy. Tracheomalacia is another important cause currently gaining recognition. Other less common causes are chronic inflammatory diseases (amyloidosis, sarcoidosis and relapsing polychondritis), infectious diseases (tuberculosis and rhinoscleroma) and collagen vascular diseases (granulomatosis with polyangitis or Wegener's granulomatosis and lupus). Lung transplant patients can present symptomatic stenosis or malacia at the site of the anastomosis. Finally, if no other cause is identified, the condition may be termed idiopathic tracheal stenosis.2,3 There are other causes of obstruction that will not be addressed in this review, such as extrinsic compression due to cervical lymphadenopathies or masses, obstruction due to benign endoluminal tumors (Fig. 1, images 8–10), radiation and inhalation lesions, and the aspiration of foreign material.
Conditions Associated With Non-malignant Airway Obstruction.
| Lymphadenopathies |
| Infectious |
| Inflammatory diseases |
| Sarcoidosis |
| Wegener's granulomatosis |
| Vascular |
| Rings |
| Anatomical variations |
| Granulation tissue |
| Endotracheal tubes |
| Tracheostomy tubes |
| Airway stents |
| Foreign material |
| Surgical anastomosis |
| Wegener's granulomatosis |
| Pseudotumor |
| Hamartomas |
| Amyloid |
| Papillomatosis |
| Hyperdynamic |
| Tracheobronchomalacia |
| Excessive pars membranosa collapse |
| Idiopathic |
| Tuberculosis |
| Sarcoidosis |
| Other |
| Goiter |
| Mucous plug |
| Vocal cords |
| Epiglottitis |
| Blood clot |
Images of different types of non-malignant obstructive airway disease. (1) Post-intubation stenosis. (2) Granulation stenosis secondary to silicone stent. (3) Tracheobronchomalacia. (4) Stenosis secondary to Wegener's granulomatosis. (5) Stenosis secondary to tuberculosis. (6) Tracheobronchopathia osteochondroplastica. (7) Idiopathic stenosis. (8) Hamartoma. (9) Solitary papilloma. (10) Papillomatois.
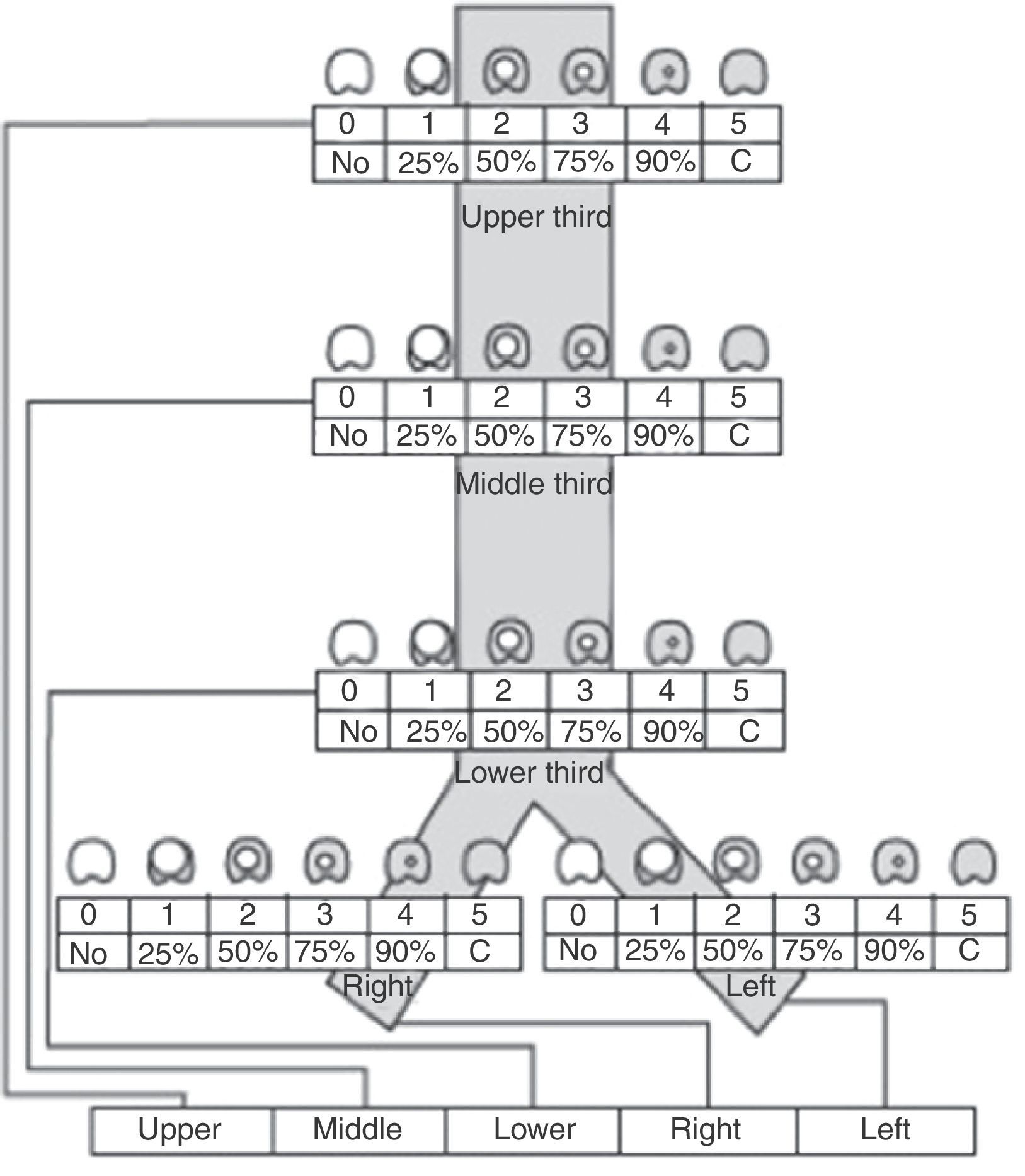
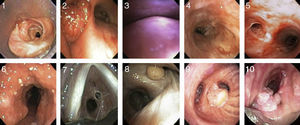
Recently, Freitag et al.4 published a classification system aiming to divide stenosis into structural and dynamic types, with additional categorization by degree of stenosis and site. Unfortunately, this classification is complex and has not been universally accepted (Fig. 2).
Stenosis classification system, according to site, grade and type of stenosis, proposed by Freitag et al.4
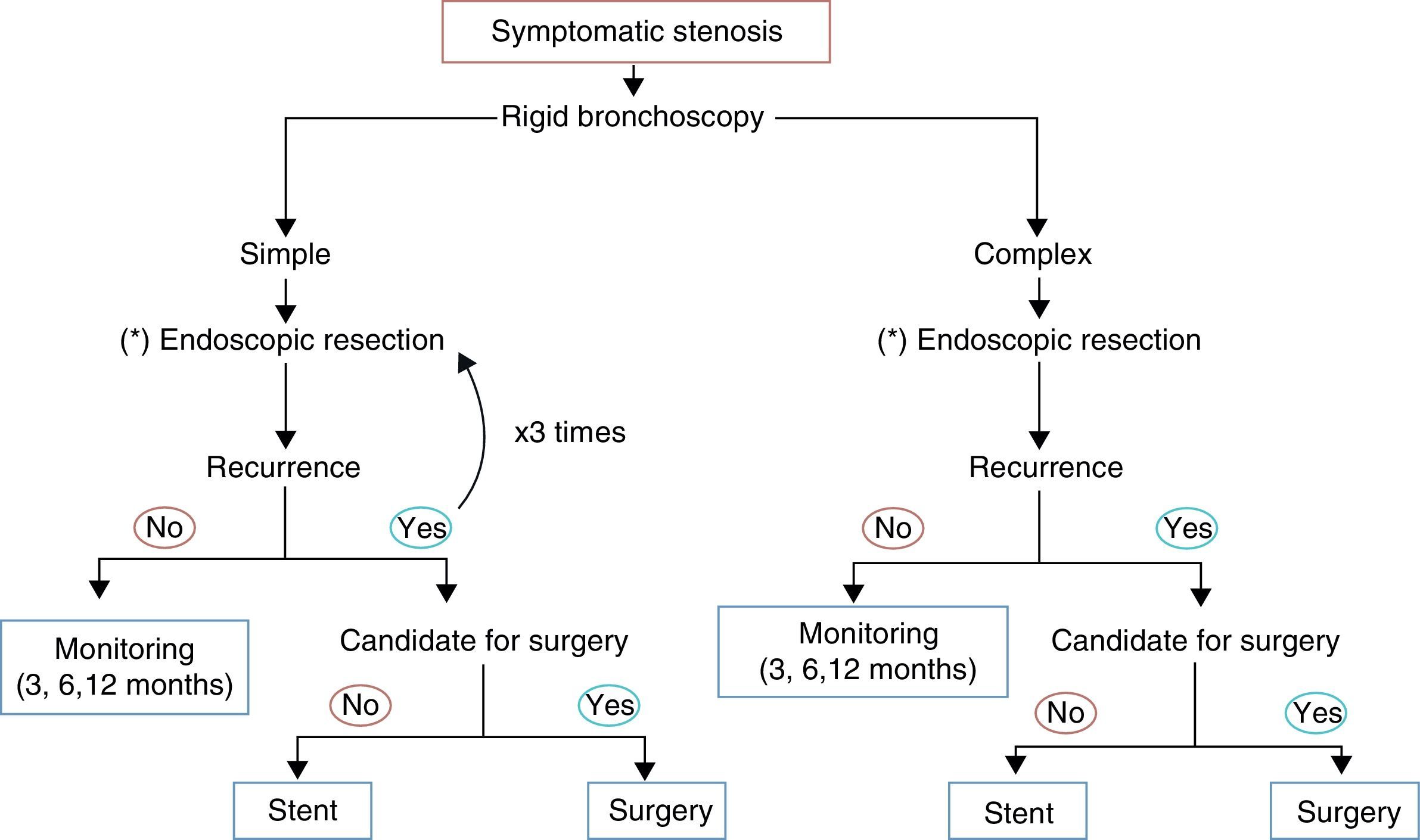
In the opinion of the authors, the most important differentiation to be made is between simple and complex stenoses, since this determines the success or failure of the endoscopic intervention. A complex stenosis is defined here as stenosis with one or more of the following characteristics: long (>10mm), tortuous, with contractions or cartilaginous damage associated with malacia. All these factors add to the difficulty of endoscopic intervention and make surgery the therapeutic method of choice.
Clinical PresentationVarying degrees of dyspnea and cough, stridor and wheezing make up the clinical spectrum of this disorder. Clinical presentation will depend not only on the underlying disease but also on the site of the lesion, the degree of narrowing of the lumen and how fast it progresses. Other factors, such as the patient's underlying state of health, may play an important role in the progress and final outcome of the process. Up to 54% of patients with tracheal stenosis initially present with respiratory distress,5 since before symptoms appear, there has been a significant and progressive loss of airway lumen diameter. Due to the similarity of the symptoms and partial response to corticosteroids, bronchodilators and antibiotics, most patients, for varying periods of time, are erroneously diagnosed with difficult-to-control asthma or recurrent chronic bronchitis. Persistent symptoms despite treatment and a strong clinical suspicion should guide the correct diagnosis.
Diagnostic EvaluationThe most commonly used diagnostic studies are pulmonary function tests, computed tomography (CT) and bronchoscopy.6
Pulmonary function tests are useful for both diagnosis and follow-up after an intervention. In the case of tracheal stenosis, a trend toward flattening of the inspiratory and expiratory flow-volume loop is observed, depending on the site and characteristics of the lesion.7 This change is not normally seen until the tracheal lumen measures less than 10mm. While variable extrathoracic obstructions show flattening of the inspiratory loop, intrathoracic obstructions show flattening of the expiratory loop. Fixed obstructions show flattening of both loops.
Extremely thin slices allowing 3-dimensional reconstructions that are highly useful for diagnostic purposes can be obtained with the multidectector CT. Dynamic CT has also been shown to be an effective and non-invasive imaging technique for the diagnosis of TBM.8–11
A direct view of the lesion can be obtained using both flexible and rigid bronchoscope images, for the evaluation of the degree of lumen narrowing, the state of the mucosa and the length, shape and distance of the stenosis from the vocal cords and the main carina. Specimens can also be obtained for microbiological culture, cytology and pathological evaluation.6,12
In many cases, a pH-meter must be used to rule out gastroesophageal reflux disease (GERD), since the association between this and laryngotracheal stenosis has been established. GERD plays an important role in the clinical course of stenosis and in persistent treatment failure, and is also associated with the idiopathic forms of tracheal stenosis.13,14
Post-Intubation and Post-Tracheotomy StenosisThe incidence of post-intubation and post-tracheotomy tracheal stenosis (PITS and PTTS, respectively) ranges from 10% to 22%,15,16 but only 1%–2% require treatment.17 At present, PITS and PTTS are recognized entities, with an incidence of 4.9 cases per million inhabitants.18
PITS occurs at the endotracheal tube cuff site in one third of cases16 (Fig. 1, image 1). The main cause appears to be the loss of local blood flow due to pressure from the cuff. This ischemia starts in the first hours after intubation and resolves with the formation of web-like fibrosis in about 3–6 weeks.19,20 Fortunately, the introduction of both low-pressure cuffs and routine monitoring has reduced the incidence of this entity.21 Web-like stenosis is the most common form of PITS.22
In contrast, PTTS occurs as a result of an abnormal tissue repair process with the formation of excessive granulation tissue around the stoma (Fig. 1, image 2) and even above or across the cartilage that was damaged during the intervention in the anterior tracheal wall.16 Many different forms of stenosis are found, including A-shaped, circumferential and granulation tissue stenoses, among others.22 They are also frequently associated with focal tracheomalacia (Fig. 1, image 3).
Other factors that have been associated with the development of PITS and PTTS are the level of the tracheotomy stoma, prolonged intubation, traumatic intubation, history of intubation or previous tracheotomy, high dose corticosteroids, advanced age, female sex, severe respiratory failure, severe GERD, concomitant autoimmune diseases, sleep apnea–hypopnea syndrome and local radiation therapy.23 There is still no consensus regarding the moment when a mechanically-ventilated patient with orotracheal intubation should undergo tracheotomy. Thus, Stauffer et al. indicate that intubation for less than 20 days is not associated with laryngotracheal complications or sequelae, and any possible complications may be due to poor technique.24 In contrast, Whited recommends that intubation should not continue for more than 5 days, reporting a high rate of laryngotracheal lesions after that time.25 In the opinion of the authors of this review, patients who require prolonged mechanical ventilation should be tracheotomized between day 7 and 14 to minimize complications secondary to intubation.
If the length of these lesions is compared, it can be seen that post-intubation stenoses have a mean length of 2.6cm, while mean lesion length post-tracheotomy is 1.2cm.26
The typical PITS or PTTS patient profile is one of an obese female smoker with diabetes mellitus, hypertension and cardiovascular disease. Obesity is associated with a larger neck circumference, increasing the risk of cartilage trauma and fracture during tracheotomy. Patients with diabetes mellitus and cardiovascular disease will have microvascular occlusion that would contribute to ischemia caused by cuff pressure during intubation.27,28
Stenosis treatment in these patients varies depending on the clinical presentation, lesion site, severity and type of stenosis, the mechanism by which it occurred and the presence of comorbidities. All these variables, together with the experience of the surgeon and endoscopist, will guide the most appropriate therapeutic approach.
The most common therapeutic endoscopic interventions at present are mechanical dilation with a pneumatic balloon, CO2 or NdYAG laser ablation and endoluminal stent placement.29
Since the pathogenic mechanism of PITS and PTTS are different, different treatments for each entity have been proposed. Zias et al. suggest that the best treatment for post-intubation stenosis consists in radial laser incisions with the aid of balloon dilation. On the other hand, they defend the use of laser ablation of the excessive granulation tissue observed in post-tracheotomy stenosis.22
Open surgery has an important role in the treatment of complex and recurrent stenoses, in which the stenosed segment is resected surgically with subsequent end-to-end anastomosis. There is no consensus, but personalized treatment in highly experienced reference centers is advocated. Grillo and Mathisen30 report a surgical mortality rate of 1.8%, but others have found rates of around 5%. Complications occur in up to 14% of cases and are related with re-stenosis, granulomas around the suture site, infections, bleeding and subcutaneous emphysema.31,32
In patients with complex stenosis who are not candidates for surgery, or in whom this option has failed, the use of silicone stents, specifically the Dumon type, is recommended.33,34
In patients with severe co-morbid conditions or those with simple stenosis, endoscopic procedures can serve as a bridge to surgery, but more importantly, they can be curative,35 and are currently becoming the initial treatment of choice. Galluccio et al. were able to definitively treat 96% of simple stenoses and 69% of complex stenoses with the use of bronchoscopic technique alone.36
In endoscopic balloon dilation, the entire force is delivered radially in order to minimize any mechanical damage to the mucosa while allowing better visual control of the procedure. It is indicated as an aid for other endoscopic techniques at various levels of the airway or as the sole technique in the case of simple, short stenoses that do not completely obstruct the airway lumen; this technique is well supported in the scientific literature.37
Laser is only useful in small, narrow lesions with a reduced vertical length and stable cartilaginous skeleton, although it is widely and generally used with equally good results and low risk in the case of larger lesions. The decannulation rate is high, surgical time is reduced, and hospital stay is short-term.38 For web-like stenosis, there is a variation of the technique that involves making radial incisions with the laser or with the electrocautery knife at 3, 9 and 12 o’clock before dilation.38–40
The microdebrider has been shown to be effective in lesions with excessive granulation tissue.41–43
Stenting is indicated in patients who do not respond to endoscopic dilation and are not candidates for surgical resection. It is important to remember that the stents indicated for this type of lesion must be easy to remove; at present, silicone stents are the most commonly used. Another alternative are fully polyurethane-coated AERO hybrid nitinol stents. These are self-expanding and can be removed, and do not require rigid bronchoscopy for implantation.44,45 Loss of cartilaginous support in the absence of extrinsic compression leads to migration of stents located in the subglottic region or proximal trachea. In these cases, external percutaneous fixation may be considered. Potential complications include skin infections around the external button.46,47 Re-stenosis as a result of the repair process itself and stent obstruction are the main reasons for re-intervention.23,39
The use of topical mitomycin is controversial, but together with radial laser incisions and balloon dilation it has some beneficial effect compared to placebo at 2–3 years48–50 (Fig. 3).
Subglottic stenosis, mainly caused by intubation, deserves a special mention. The subglottic space refers to the section of the airway between the vocal cords and the lower fraction of the cricoid cartilage, which is the narrowest section of the larynx and the only one surrounded by a complete ring of cartilage. Its narrow diameter, inextensibility of the surrounding tissue, fragility of the coating tissue and poor vascularization make it more susceptible to trauma from intubation, re-stenosis and failure to decannulate.51 An incidence of subglottic stenosis secondary to prolonged intubation in children and adults ranging from 0.9% to 8.3% has been reported.52 Management is a challenge involving various strategies that must be tailored to suit each patient. For non-concentric soft, membranous stenoses with sufficient cartilaginous support and a length of around one centimeter corresponding to Cotton-Meyer grades I and II, endoscopic techniques described above are used, with emphasis on the use of laser. The success rate is variable according to the literature, ranging between 40% and 94%.53 Longer, hard, grade III and IV complex stenoses can be treated initially with endoscopic techniques, but in most cases, open reconstructive surgery will be required (surgical resection of the stenosed section, including several tracheal rings and the anterior cricoid ring, in addition to the lower half of the mucosa of the cricoid cartilage, followed by end-to-end anastomosis).53,54
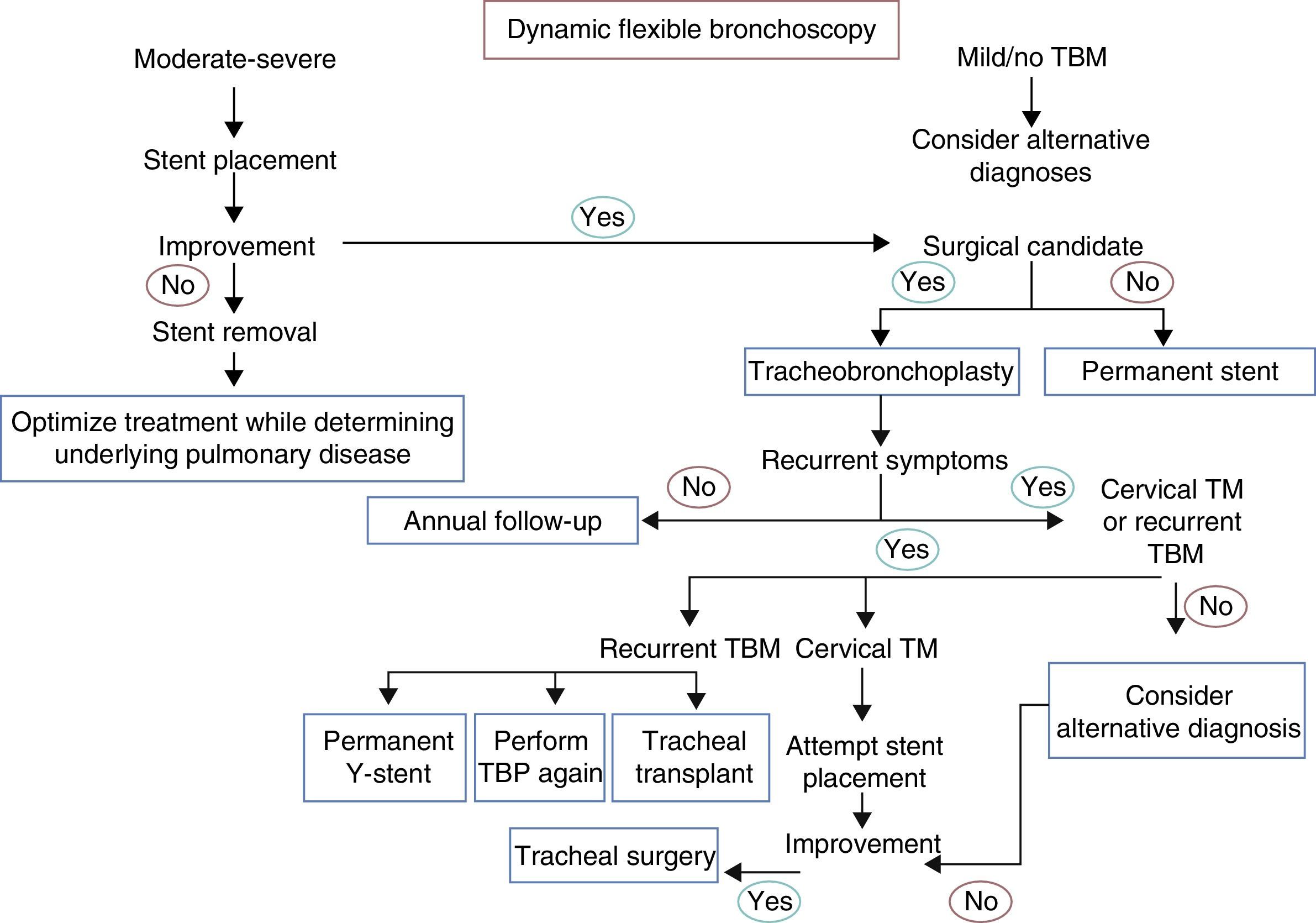
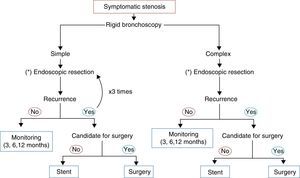
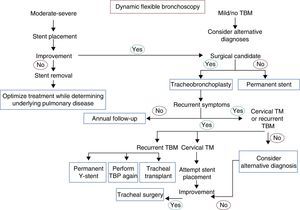
Dynamic Airway Obstruction: Tracheobronchomalacia and Excessive Pars Membranosa CollapseTBM and excessive pars membranosa collapse occur in around 12% of patients with respiratory diseases.55 In TBM, the proportion between cartilage and soft tissues is reduced from a normal ratio of 5:1 to 2:1, while in excessive pars membranosa collapse, there is atrophy and a loss of myoelastic fibers.56 TBM, in both its local and diffuse forms, may be caused by various factors57,58 (Table 2). There are different ways of classifying the disease, but the functional classification (FEMOS) is the most comprehensive.59 TBM may be asymptomatic, although it often produces cough, wheezing, stridor, dyspnea, recurrent infections, and on occasions, respiratory failure,60 and therefore differential diagnosis is needed to rule out disease entities such as chronic obstructive pulmonary disease, asthma and bronchiectasis.61 Respiratory function tests can help in the diagnosis of concomitant obstructive pulmonary disease, but they have limited application in the diagnosis of TBM, since results are normal in up to 21% of cases.62 Accordingly, dynamic chest tomography and dynamic flexible bronchoscopy are often required for diagnosis62,63 (Fig. 1, image 3). This disease can be easily diagnosed by the performance of dynamic inhalation and exhalation maneuvers. In patients with diffuse TBM, a diagnostic test must be performed with silicone stent placement,61,64 along with management of comorbidities. Patients who show improvement in their symptoms will have the stent removed in preparation for surgical reconstruction by tracheobronchoplasty.65 Patients who cannot undergo surgery due to their comorbidities will be managed with a combination of symptomatic treatment and possible definitive stenting (Fig. 4).
Classification of Most Common Causes of Tracheobronchomalacia in Adults.
| Primary |
| Genetic |
| Idiopathic |
| Tracheobronchomegalia (Mounier–Kuhn syndrome) |
| Secondary |
| Post-traumatic |
| Post-intubation |
| Post-tracheotomy |
| Chest trauma |
| Post-lung transplantation |
| Emphysema (COPD) |
| Chronic bronchitic infections |
| Chronic inflammation |
| Relapsing polychondritis |
| Extrinsic tracheal compression |
| Benign tumors |
| Malignant tumors |
| Cysts |
| Abscesses |
| Aortic aneurysm |
| Vascular rings or abnormalities |
Flowchart for the management of tracheobronchomalacia. TBM: tracheobronchomalacia; TBP: tracheobronchoplasty; TM: tracheomalacia. Annual follow-up: dynamic computed tomography, dynamic bronchoscopy, pulmonary function tests. Alternative diagnosis: asthma, gastroesophageal reflux disease, vocal cord dysfunction, immunodeficiencies.
Although non-invasive ventilation has been proposed as a possible treatment for TBM, its role appears to be restricted to the management of acute respiratory failure in TBM post-intubation, since it keeps the airway open and allows drainage of secretions. In this respect, Murgu and Colt have recently proposed diagnostic bronchoscopy via the continuous positive airway pressure (CPAP) interface, provided the patient is not in a critical situation, with the aim of determining if the patient would indeed respond to and benefit from positive airway pressure.66 If the patient is stable, intermittent nasal pressure during the day and continuous pressure at night is recommended. This stabilizes the patient's airway and acts as a bridge to more specific and definitive treatments, such as stent implantation or surgery (tracheobronchoplasty).67 CPAP appears to circumvent the need for tracheotomy or prolonged intubation in cases of mild to moderate TBM.68
The human trachea is a unique and complex organ that requires rigid support to withstand the respiratory cycle, adequate vascular support for maintenance, and an epithelium that makes it resistant to aggressions from the external environment. In this respect, the flowchart for the management of persistent TBM after tracheobronchoplasty includes the possibility of performing tracheal transplantation. This is a novel treatment modality that is still under evaluation; results are uncertain, and few cases have been studied. It is reserved for very specific situations that require the resolution of post-surgical problems (as would be the case here) or a possible alternative to tracheobronchoplasty itself. Delaere, albeit outside the scope of TBM, proposed tracheal allotransplantation with temporary immunosuppression. The procedure consists in implantation of a trachea from a cadaver donor after heterotopic revascularization for 3 months on the forearm of the recipient, in which the tracheal epithelium was finally replaced by buccal mucosa in order to prevent rejection and facilitate definitive cessation of immunosuppressive treatment.69
Tracheobronchial Stenosis in Granulomatosis With Polyangitis (Wegener's Granulomatosis)The airway is involved in 15%–55% of cases.70–72 This is the only manifestation in up to 25% of patients,73 and can even be irreversible. Respiratory manifestations include obstruction and/or necrosis of the nasal cartilage, subglottic stenosis, tracheal and bronchial stenoses, malacia, membrane formation, nodules and masses, alveolar infiltrates and cavitations74,75 (Fig. 1, image 4). Patients are usually young, under the age of 30, and mainly female.76,77 The main symptoms are cough, wheezing, dyspnea, stridor and hemoptysis.72,78 Involvement of the posterior tracheal wall is common, unlike in other disorders such as relapsing polychondritis or tracheobronchopathia osteochondroplastica.79 Subglottic stenosis is the most common endobronchial manifestation in Wegener's granulomatosis80,81 and there is usually no correlation between inflammatory activity in the airway (seen on biopsy) and positive c-ANCAs.82–86
Endoscopic treatment includes injection of corticosteroids into the lesion, pneumatic balloon dilation and thermoablation. The use of stents and tracheotomy must be avoided, since these procedures have their own complications. Generally surgical resection with re-anastomosis is used in highly selected cases.78,79,87–92
Intralesional application of long-acting corticosteroids (60–80mg methylprednisolone acetate) together with endoscopic dilation appears to be an effective treatment.93–95
Tracheobronchial Stenosis in AmyloidosisSubglottic obstruction is the most common form (0.5% of all symptomatic lesions in the tracheobronchial tree and 23% of all benign symptomatic lesions). Simultaneous involvement of the parenchyma and the tracheobronchial tree is uncommon.96
Amyloidosis in the tracheal mucosa can cause disease ranging from diffuse lesions to masses simulating tumors.97 Diagnosis is determined when a biopsy of the lesion shows red Congo staining with apple-green birefringence under polarized light. Irregular narrowing of the lumen, wall thickening and irregular calcifications can be observed on endoscopy.96 Some patients can have airway obstruction or hemoptysis: in these cases, laser is the treatment of choice.98–100 In patients with diffuse disease, Kurrus et al. documented the regression of endobronchial amyloid deposits after 10 radiotherapy sessions of 20Gy each.101
Tracheobronchial Stenosis Due to TuberculosisAn endobronchial component is present in 10%–40% of active pulmonary tuberculosis,102,103 with involvement of the primary bronchi in 60%–95% of cases.104 This is most frequently seen when diagnosis and treatment are delayed.105,106 The most likely cause is lymph node involvement with subsequent fistulization toward the adjacent bronchi.107 Endobronchial tuberculosis can present as a caseous/edematous, hyperemic, fibrostenotic, granular, tumor or ulcerative lesion.105,108 It often presents as a white, gelatinous, polypoid lesion (Fig. 1, image 5).
Endoscopic treatment includes thermoablation and serial balloon dilations. Stent implantation can be considered for symptomatic irreversible scar lesions or extrinsic compression of the airway.109
Tracheobronchial Stenosis in Tracheobronchopathia OsteochondroplasticaTracheobronchopathia osteochondroplastica is a rare, non-tumorous disease that affects the trachea and to a lesser extent the primary bronchi, presenting as submucous nodules of cartilaginous or bony origin projecting into the airway lumen.110–113 These nodules can be of different sizes but generally measure between 1mm and 3mm and are located in the anterolateral tracheal wall with no posterior wall involvement.114 They can cause deformity and narrowing of the trachea, although in only 10% of cases do they occupy more than 50% of the lumen.110
Higher than normal concentrations of certain cytokines (BMP-2, TGF-B1) have led to the suggestion that this disorder may be the result of metaplasia of the mesenchymal connective tissue adjacent to the submucosa.115
This disease is not associated with smoking, and prevalence does not differ between men and women. The majority of cases are diagnosed in middle-aged subjects.116,117
CT reveals densely calcified nodules in the submucosa protruding into the anterolateral wall of the airway lumen.110,112,114 These same findings are confirmed on bronchoscopic visualization (Fig. 1, image 6). If the appearance is typical, no biopsy is necessary. If biopsy is performed, bronchial submucosa is found to be bony or calcified.110 Tracheobronchopathia osteochondroplastica is a slow, benign disease that rarely causes complications such as post-obstructive pneumonias or respiratory failure.116 If obstructive symptoms are present, most patients are treated with endoscopic laser ablation and stents.110,113,116 Surgical resection is rarely required.
Idiopathic Tracheal StenosisIn most cases, this type of stenosis is located in the subglottic region or in the upper third of the trachea.118–120 It occurs mainly in women, suggesting that estrogens have an important role in this entity.118–121 Other authors suggest that it may be associated with GERD.122,123
Although evaluation of the flow-volume loop may suggest the diagnosis, multi-slice CT and bronchoscopy (Fig. 1, image 7) are essential for confirmation.124–126
Histological specimens retrieved during bronchoscopy reveal dense fibrosis and moderate inflammatory infiltration with a significant amount of fibroblast formation.127
While surgery remains the definitive treatment,48,128,129 lesions smaller than 1cm can be successfully treated with endoscopy techniques, performing radial incisions followed by balloon dilation and the topical application of mitomycin C. The use of removable stents can be considered in patients with recurrent lesions who are not candidates for surgery or as a bridge to surgical intervention. For simple stenoses, at least three bronchoscopic sessions are recommended before surgery is considered. Injection of steroids into the lesion or the application of mitomycin C has been used to prevent re-stenosis after endoscopic treatment.120,48,130 Surgical resection is the treatment of choice for complex stenotic lesions.
Tracheobronchial Stenosis in Relapsing PolychondritisRelapsing polychondritis is a multi-system autoimmune disease with recurrent inflammatory episodes affecting cartilaginous structures, such as the ears, nose, peripheral joints, larynx and tracheobronchial tree.131,132 It is more common between the fourth and fifth decades of life, and is not gender-predominant.131
During the course of this disease, approximately half of patients will have pulmonary and airway involvement, including for example, subglottic stenosis, focal or diffuse malacia and tracheobronchial stenosis. Dynamic chest CT with slices obtained in inspiration and forced expiration is the imaging test of choice. Focal stenosis, thickening of the tracheal wall with or without calcifications and expiratory collapse associated with concentric malacia may be observed.133 PET imaging may be useful for diagnosis and for evaluating response to treatment.134,135
Some patients require interventions such as balloon dilation, stents or tracheotomy. In those in whom TBM is an added factor, intermittent CPAP, expectorants and flutter valves may be used to avoid mucostasis and superinfection. Medical treatment generally consists of anti-inflammatory treatment with corticosteroids combined with methotrexate, azathioprine or cyclophosphamide.131 Some studies support the use of new immunomodulatory therapies, such as etanercept, infliximab and rituximab.136–140
Tracheobronchial Stenosis in SarcoidosisThe airway may be compromised even in the absence of parenchymal involvement.141–143
The formation of granulomas gives the mucosa a cobblestone appearance.141 Other forms of involvement are erythema, edema and plaque formation. Narrowing of the airway secondary to scar stenosis or extrinsic compression due to mediastinal lymphadenopathies is rare.144
Cough is the most common clinical manifestation of this disease when it presents in the main airway.141,145–149 Endoscopic findings range from single or multiple stenoses to diffuse airway narrowing.141,150,151
In patients with mild symptoms, inhaled corticosteroids should be sufficient treatment, but systemic corticosteroids may be added.141 Bronchoscopic procedures, for example, pneumatic dilation and thermoablation, are required in some cases, in addition to attempts with intralesional corticosteroids.
Post-lung Transplantation Bronchial StenosisPost-transplantation bronchial stenoses are a significant source of morbidity and mortality, and are the result of the anastomosis repair process. It occurs at a rate of between 16% and 33%152 and mortality ranges between 2% and 4%.153–159
These stenoses are vulnerable to ischemia, since the circulation of the bronchial arteries is not generally immediately re-established and perfusion depends on retrograde flow from the pulmonary artery until a collateral flow is established after a period of 2–4 weeks.160 Other factors such as rejection, immunosuppressive treatment, infections or inadequate organ preservation have been involved in changing the course of the repair process.161,162 Since two thirds of these patients have concomitant bronchomalacia, pneumatic balloon dilation is usually a temporary solution, and stents are required in most cases.163–166 De Gracia et al. have reported that stents are required in only half of cases.167 Silicone or hybrid stents166 must be used only for recurrent stenoses that have not responded to 3–4 balloon dilations or in cases of severe symptomatic focal malacia.165
Recently Dutau et al., in a series of 17 cases, proposed the use of silicone stents, pointing out the resolution of stenosis and healing of the anastomosis in most patients (with fewer side effects than the self-expanding metallic stents generally used), after which the stent can be removed.168 One of the major problems encountered is the location of the anastomosis sutures that generally make it difficult to adapt the stent to the anatomy of the patient, resulting in migration and/or obstruction of the entrances to the upper lobes. These complications, particularly in the case of stenosis of the intermediate bronchus, appear to be resolved by placing the tracheotomy arm of a modified Montgomery T-tube at the entrance to the right upper lobe, thus maintaining patency.169
ConclusionBenign central airway lesions frequently call for therapeutic bronchoscopy procedures. Treatment of these disorders requires immediate stabilization, detailed evaluation, meticulous planning and tailored treatment. An evaluation of each lesion that encompasses physiopathology and the natural history of the disease is required. Treatment must be planned by a multidisciplinary team that includes interventional pulmonologists, chest surgeons, anesthetists, ear, nose and throat specialists and radiologists. In practice, therapeutic bronchoscopy and tracheal surgery are interrelated, complementary procedures.
Conflict of InterestsNone.
FundingNone.
None.
Please cite this article as: Barros Casas D, Fernandez S, Folch E, Flandes Aldeyturriaga J, Majid A. Patología obstructiva no maligna de la vía aérea central. Arch Bronconeumol. 2014;50:345–354.