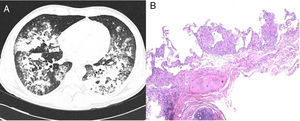
We report the case of a 42-year-old man, Dutch national, resident in Spain for 11 years. He was an active smoker with a cumulative pack-year index of 15, and no other toxic habits, and reported no use of saunas or hot tubs. He had a diagnosis of pityriasis lichenoides, treated with 7.5mg methotrexate weekly for the previous 5 years. He attended the emergency department with a 2-week history of 38°C fever, myalgia, cough, expectoration of mucus, and dyspnea on moderate exertion. Initial clinical laboratory results showed 11,190 leukocytes/mm3 (70% neutrophils, 19% lymphocytes, 9% monocytes), C-reactive protein 11.2mg/dl, and procalcitonin 0.56ng/ml. Arterial blood gases were FiO2 0.24, pH 7.48, PaCO2 37mmHg, PaO2 87mmHg and HCO3 27mmol/l. Chest X-ray revealed bilateral consolidations in the middle and lower fields. Urine antigen testing was negative for Streptococcus pneumoniae and Legionella pneumophila serogroup-1, and nasopharyngeal swabs for H1N1 virus, sputum smear microscopy and HIV serology were also negative. Community-acquired pneumonia with Fine score III1 in an immunosuppressed patient was suspected, so antibiotic therapy with ceftriaxone and levofloxacin was started, and the patient was admitted to the respiratory medicine ward. Three days after admission, the patient's fever persisted with no clinical improvement, so the first bronchoscopy was performed, revealing a predominantly mononuclear cell count in the bronchoalveolar lavage (BAL): 58% macrophages, 60% lymphocytes, 3% neutrophils. Only adenovirus was identified by polymerase chain reaction (PCR), so cidofovir was added to the treatment. One week after hospitalization, the patient's failure to improve clinically and radiologically prompted the performance of a chest computed tomography (CT) which confirmed consolidation in both lung bases (Fig. 1A), so a second bronchoscopy was performed and a transbronchial biopsy was obtained. Tests for anti-nuclear and anti-neutrophil cytoplasmic antibodies, rheumatoid and angiotensin-converting enzymes were all negative. Two days later, the patient presented clinical worsening with progressive consolidation and high oxygen requirements (PaO2/FiO2: 97mmHg), so he was transferred to the Intensive Care Unit for monitoring and administration of high-flow oxygen therapy. Standard cultures of both bronchial aspirate and BAL grew saprophytic flora, and the sputum smear, galactomannan and study of viruses and parasites were all negative. BAL cytology showed persistent predominance of mononuclear cells. Transbronchial biopsy revealed non-necrotizing granulomas (Fig. 1B). Mycobacterial infection was suspected, although other etiologies such as sarcoidosis or pneumonitis associated with methotrexate could not be ruled out. Standard anti-tuberculosis treatment and methylprednisolone (1mg/kg/day) were started. PCR testing (Real-Cycler TBM, Molecular Progeny) for Mycobacterium tuberculosis detection in BAL and the biopsy specimen was requested, both of which were negative. Having ruled out tuberculosis, a PCR was performed for non-tuberculous mycobacteria (NTM) (INNO-LIPA Mycobacteria v2, Innogenetics) on the first BAL sample, in which Mycobacterium intracellulare (M. intracellulare) and Mycobacterium simiae (M. simiae) were identified. Treatment was switched to rifampicin, ethambutol, azithromycin, and moxifloxacin, and rapid tapering of the corticosteroids began. Clinical and radiological improvement was observed, with oxygen saturation of 94% breathing room air at discharge. No microbiological confirmation was obtained from mycobacterial cultures in any of the respiratory samples tested. The patient continued treatment for 1 year, and remained asymptomatic with practically complete resolution of radiological changes.
The worldwide incidence and prevalence of pulmonary disease caused by NTM is increasing. It affects both immunosuppressed and immunocompetent individuals2; in our case, a patient receiving chronic treatment with methotrexate.
With regard to the mycobacteria identified in our patient, M. simiae is rarely associated with lung disease, suggesting that its isolation is due to environmental contamination.3 Indeed, pseudo-outbreaks caused by contaminated hot water supplies have been reported.4 Most cases have been described in the southern United States, Cuba, and Israel.3–5 Affected patients are primarily elderly immuno competent individuals with underlying lung disease, but disseminated infection can also occur in patients with advanced HIV infection (Aids).6 Treatment is complicated by the lack of correlation between in vitro susceptibility and the in vivo response, but must include clarithromycin in combination with other antimicrobials, such as fluoroquinolones and cotrimoxazole.3,6M. intracellulare, on the other hand, is included in the Mycobacterium avium (M. avium) complex. This species, unlike M. avium, is isolated more frequently in patients without HIV infection and presents with two main radiological patterns: fibrocavitary disease and nodular bronchiectasis.3 Cases have been reported in the United States, Japan, Europe, and South Africa.7 It is resistant to chlorine and survives at high temperatures, so hot tubs or jacuzzis are associated with outbreaks.8 Treatment must include a macrolide, rifampicin, ethambutol, and, if cavitary or disseminated lesions are observed, an injectable aminoglycoside during the induction phase is required.3
Methotrexate has anti-proliferative and immunosuppressive activity, and is one of the therapeutic options for the treatment of pityriasis lichenoides.9 Chronic use can lead to immune changes that predispose to opportunistic infections.10–12
This is the first report in the literature of mycobacterial infection caused by M. simiae and M. intracellulare during chronic treatment with methotrexate. Our patient met the ATS/IDSA criteria3 for the diagnosis of pulmonary disease caused by NTM, guided by the finding of granulomatous lesions in the biopsy and identification by molecular techniques. The patient also improved clinically and radiologically with treatment targeted at both microorganisms. However, the pathogenic role of M. simiae may be questionable, since previous studies have suggested that only 20% of isolates in respiratory samples are clinically relevant.13 We cannot determine the influence that the initial treatment, including levofloxacin, may have had on the failure to grow M. simiae and M. intracellulare in the mycobacterial cultures, and the molecular biology techniques were objectively more sensitive for diagnosis.
In conclusion, NTM infections should be considered in the differential diagnosis of pneumonia in patients receiving immunosuppressive therapy.
We thank Dr. Lara Pijuan of the Pathology Department of the Hospital del Mar and Dr. Ana C. Siverio Parés of Microbiology Reference Laboratory of Catalonia.
Please cite this article as: Dal Molín-Veglia MA, Sánchez-Martínez F, Fernández-Alarza AF, Domínguez-Álvarez M. Micobacteriosis pulmonar en un paciente en tratamiento crónico con metotrexato. Arch Bronconeumol. 2018;54:225–226.