Persistent thrombotic lesions are common in patients with pulmonary embolism. These lesions occur on a clinical spectrum, ranging from an asymptomatic course with complete functional recovery to chronic thromboembolic pulmonary hypertension. The concept of chronic thromboembolic disease has emerged in recent years to describe a subgroup of patients with persistent thrombotic lesions who have symptoms on exertion and pulmonary vascular dysfunction, but no pulmonary hypertension at rest. The prevalence of this entity is unknown and the criteria for diagnosing it are not defined. The aim of this article is to analyze post-pulmonary embolism sequelae and review existing evidence on chronic thromboembolic disease, with special emphasis on its diagnosis and therapeutic approach.
Es frecuente observar lesiones trombóticas persistentes en los pacientes que sufren una embolia pulmonar. Estas lesiones pueden cursar con un espectro clínico amplio, desde un curso asintomático con recuperación funcional completa hasta la hipertensión pulmonar tromboembólica crónica. En los últimos años ha emergido el concepto de enfermedad tromboembólica crónica pulmonar para designar al subgrupo de pacientes con lesiones trombóticas persistentes que presentan síntomas con el esfuerzo y disfunción vascular pulmonar, pero que no muestran hipertensión pulmonar en reposo. La prevalencia de esta entidad es desconocida y los criterios para diagnosticarla no están definidos. El objetivo del presente artículo es analizar las secuelas que se producen tras una embolia pulmonar y revisar la información disponible sobre la enfermedad tromboembólica crónica, con especial énfasis en su diagnóstico y abordaje terapéutico.
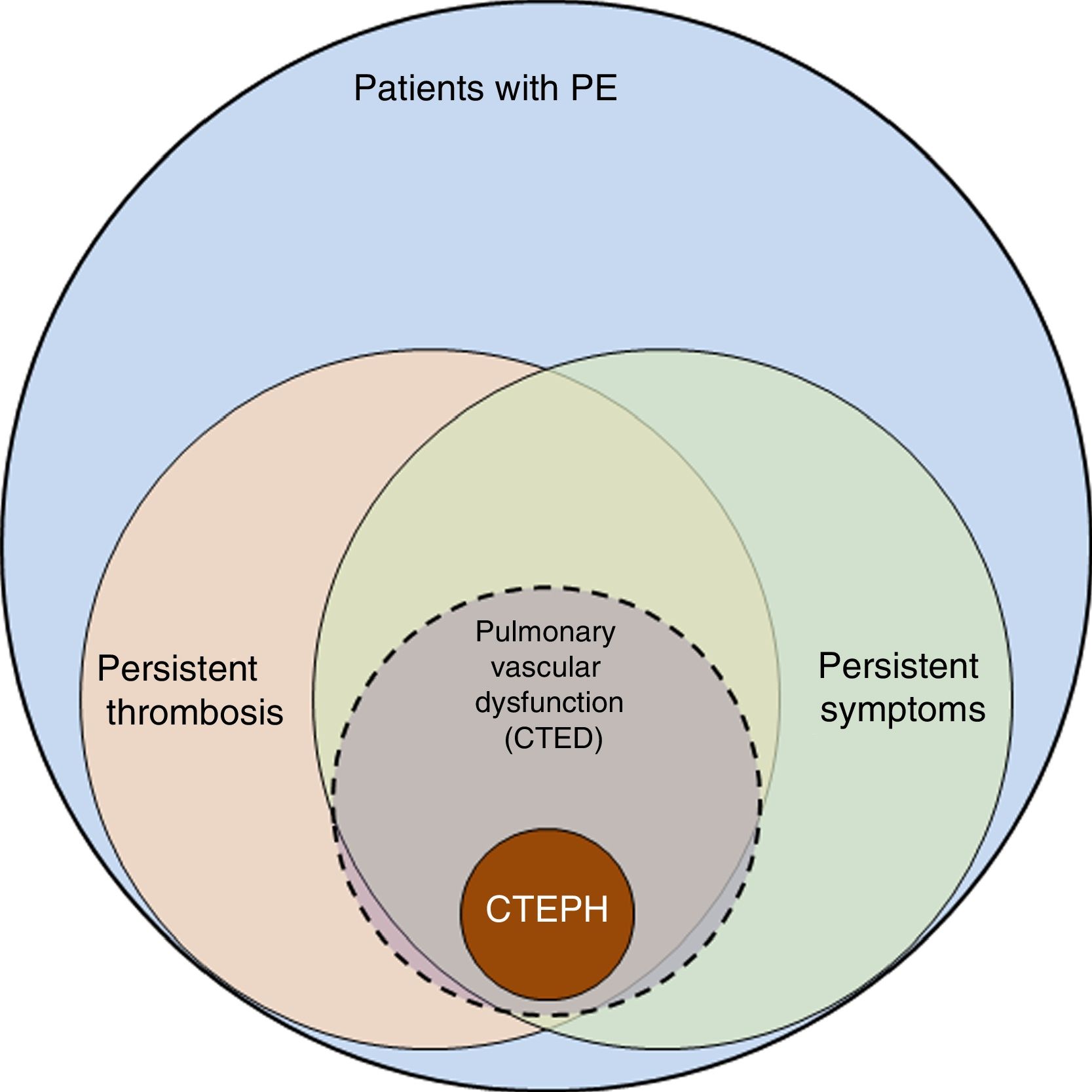
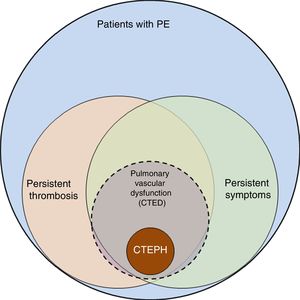
The incidence of pulmonary embolism (PE) in Spain has risen from 20.4 cases per 100 000 population/year in 2002 to 32.7 in 2011. Patients are becoming increasingly complex, with a higher number of PE-related comorbidities, thus increasing treatment costs, albeit with lower mortality.1 These findings suggest improvements in the diagnosis and treatment of PE, but they also highlight the challenge involved in follow-up of these patients, since the number of survivors who may present sequelae after acute PE has grown. The spectrum of these sequelae ranges from persistent asymptomatic pulmonary thrombosis to chronic thromboembolic pulmonary hypertension (CTEPH) (Fig. 1). CTEPH, the most serious complication following PE, is well defined and its diagnosis and treatment clearly established.2,3 However, the definition of CTEPH does not include a subgroup of patients who report dyspnea on exertion and/or functional limitation that they did not have prior to the PE, and who present residual thrombosis but normal pulmonary arterial pressure (PAP) at rest. In these patients, the obliteration produced by persistent thrombosis is considered to cause pulmonary vascular dysfunction, which manifests primarily as exercise intolerance.4 The pathophysiological mechanism of this dysfunction, its clinical and therapeutic implications, as well as the risk factors that determine failure of thrombus resolution are not well established and are currently under investigation. Recognition of this patient subgroup has led to define a new entity known as pulmonary chronic thromboembolic disease (CTED).
Spectrum of complications after pulmonary embolism. Graphic representation of the spectrum of complications after pulmonary embolism (PE), from persistent thrombosis to chronic thromboembolic pulmonary hypertension (CTEPH). Patients with persistent thrombosis include a subgroup who present symptoms attributable to pulmonary vascular dysfunction, defined as failure of the pulmonary vascular bed to adapt the changes that occur during exercise. These patients are considered to have pulmonary chronic thromboembolic disease (CTED).
This review analyzes the spectrum of sequelae that may arise after PE, ranging from asymptomatic residual pulmonary thrombosis to CTEPH, and sets out the basis for considering CTED as a distinct clinical entity, its diagnosis and eventual treatment.
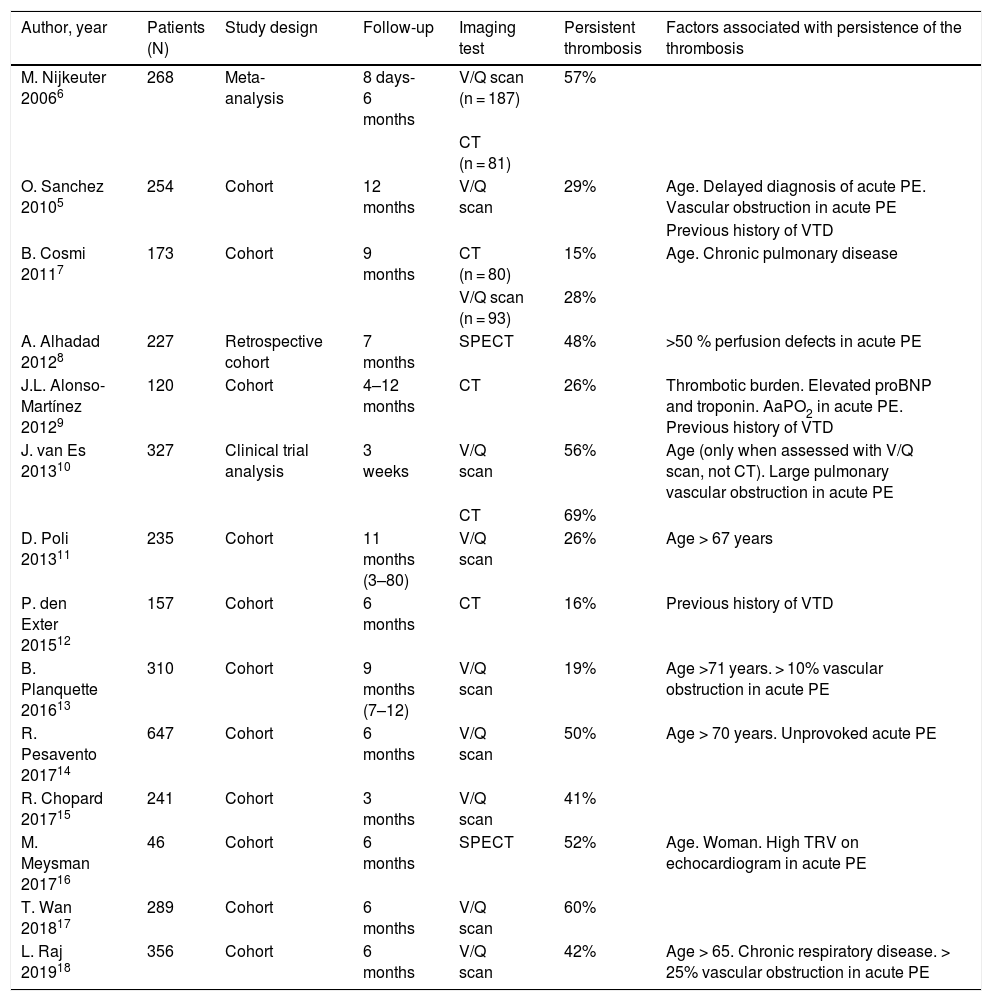
Residual thrombosisThe concept of residual or persistent thrombosis has not been clearly defined. Recanalization of a thrombus within a blood vessel is a time-dependent, dynamic process. The prevalence of residual thrombosis varies according to when it is studied after the PE, although the point at which thrombi should be considered residual has not yet been determined. The diagnostic tool used also affects the number of residual thrombi observed.5Table 1 summarizes the studies published in this regard.5–18
Persistence of residual thrombosis after acute pulmonary embolism.
| Author, year | Patients (N) | Study design | Follow-up | Imaging test | Persistent thrombosis | Factors associated with persistence of the thrombosis |
|---|---|---|---|---|---|---|
| M. Nijkeuter 20066 | 268 | Meta-analysis | 8 days-6 months | V/Q scan (n = 187) | 57% | |
| CT (n = 81) | ||||||
| O. Sanchez 20105 | 254 | Cohort | 12 months | V/Q scan | 29% | Age. Delayed diagnosis of acute PE. Vascular obstruction in acute PE |
| Previous history of VTD | ||||||
| B. Cosmi 20117 | 173 | Cohort | 9 months | CT (n = 80) | 15% | Age. Chronic pulmonary disease |
| V/Q scan (n = 93) | 28% | |||||
| A. Alhadad 20128 | 227 | Retrospective cohort | 7 months | SPECT | 48% | >50 % perfusion defects in acute PE |
| J.L. Alonso-Martínez 20129 | 120 | Cohort | 4–12 months | CT | 26% | Thrombotic burden. Elevated proBNP and troponin. AaPO2 in acute PE. Previous history of VTD |
| J. van Es 201310 | 327 | Clinical trial analysis | 3 weeks | V/Q scan | 56% | Age (only when assessed with V/Q scan, not CT). Large pulmonary vascular obstruction in acute PE |
| CT | 69% | |||||
| D. Poli 201311 | 235 | Cohort | 11 months (3–80) | V/Q scan | 26% | Age > 67 years |
| P. den Exter 201512 | 157 | Cohort | 6 months | CT | 16% | Previous history of VTD |
| B. Planquette 201613 | 310 | Cohort | 9 months (7–12) | V/Q scan | 19% | Age >71 years. > 10% vascular obstruction in acute PE |
| R. Pesavento 201714 | 647 | Cohort | 6 months | V/Q scan | 50% | Age > 70 years. Unprovoked acute PE |
| R. Chopard 201715 | 241 | Cohort | 3 months | V/Q scan | 41% | |
| M. Meysman 201716 | 46 | Cohort | 6 months | SPECT | 52% | Age. Woman. High TRV on echocardiogram in acute PE |
| T. Wan 201817 | 289 | Cohort | 6 months | V/Q scan | 60% | |
| L. Raj 201918 | 356 | Cohort | 6 months | V/Q scan | 42% | Age > 65. Chronic respiratory disease. > 25% vascular obstruction in acute PE |
AaPO2: alveolar-arterial oxygen tension gradient; BNP: brain natriuretic peptide; CT: computed tomography; PE: pulmonary embolism; SPECT: single photon emission tomography; TRV: tricuspid regurgitation velocity; VTD: venous thromboembolic disease; V/Q scan: lung ventilation-perfusion scan.
The prevalence of residual thrombosis 6 months post-PE ranges from 16% to 69%. Ventilation/perfusion scintigraphy (V/Q scan) has higher sensitivity than computed tomography (CT) angiography for the detection of persistent pulmonary thrombosis, and is thus considered the first-line imaging test.19 This high sensitivity explains the higher percentage of diagnoses with this technique, and contrasts with diagnostic studies of acute PE, where the V/Q scan does not provide greater diagnostic sensitivity than CT angiography.20
Several factors may contribute to the persistence of pulmonary thrombosis (Table 1). Recent studies have identified several independent predictors for residual thrombosis after unprovoked acute PE following 6 months of anticoagulant treatment,18 including age over 65 years, smoking and chronic respiratory disease.21,22 Elevated factor VIII levels18 and extensive vascular obstruction (>25%) in the acute PE episode are also predictors of persistent thrombosis.5,8,13
Special mention should be made of the possible clinical implications of the duration of anticoagulant therapy on residual thrombosis. The number of patients treated correctly in most CTEPH incidence studies is unknown, but patients inadequately treated with anticoagulants are assumed to be more likely to develop CTEPH.23 In other studies, persistent thrombosis is associated with longer anticoagulation time,6,24 although this may be due to a confounding effect; patients who have been on anticoagulants for longer are those with persistent dyspnea, which may be a consequence of the residual thrombosis or of cardiorespiratory comorbidities, likewise associated with pulmonary perfusion defects.
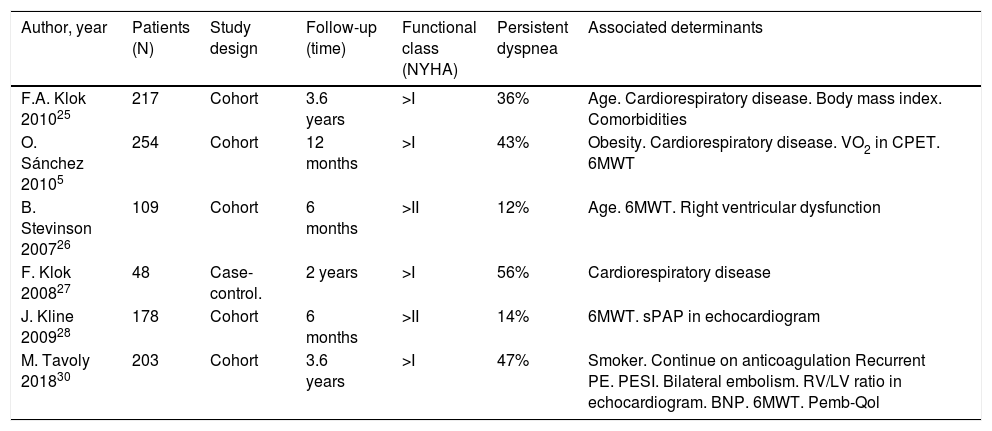
Persistent symptoms after pulmonary embolismSeveral studies show that a number of patients report worsening or persistence of their baseline dyspnea between 6 months and 3 years after an acute PE episode (Table 2).5,25–29 According to Klok et al.,25 more than one-third of patients report dyspnea 3.5 years after acute PE, and up to 76% of these report new-onset or worsened dyspnea after the episode.25 Sanchez et al.5 found that 30% of patients had persistent perfusion defects after PE, a finding that was associated with worsening dyspnea and shorter distance covered during the 6-minute walk test (6MWT), as well as an increase in the systolic PAP (sPAP) estimated by echocardiography.5
Persistent dyspnea after pulmonary embolism.
| Author, year | Patients (N) | Study design | Follow-up (time) | Functional class (NYHA) | Persistent dyspnea | Associated determinants |
|---|---|---|---|---|---|---|
| F.A. Klok 201025 | 217 | Cohort | 3.6 years | >I | 36% | Age. Cardiorespiratory disease. Body mass index. Comorbidities |
| O. Sánchez 20105 | 254 | Cohort | 12 months | >I | 43% | Obesity. Cardiorespiratory disease. VO2 in CPET. 6MWT |
| B. Stevinson 200726 | 109 | Cohort | 6 months | >II | 12% | Age. 6MWT. Right ventricular dysfunction |
| F. Klok 200827 | 48 | Case-control. | 2 years | >I | 56% | Cardiorespiratory disease |
| J. Kline 200928 | 178 | Cohort | 6 months | >II | 14% | 6MWT. sPAP in echocardiogram |
| M. Tavoly 201830 | 203 | Cohort | 3.6 years | >I | 47% | Smoker. Continue on anticoagulation Recurrent PE. PESI. Bilateral embolism. RV/LV ratio in echocardiogram. BNP. 6MWT. Pemb-Qol |
BNP: brain natriuretic peptide; CPET: cardiopulmonary exercise test; NYHA: New York Heart Association; Pemb-Qol: pulmonary embolism quality of life questionnaire; PESI: Pulmonary embolism score index; RV/LV: right ventricle/left ventricle diameter ratio; sPAP: systolic pulmonary arterial pressure; VO2: peak oxygen uptake; 6MWT: 6-min walk test.
The worsening or onset of exertional dyspnea after PE has also been evaluated using quality of life questionnaires,29,30 with worse scores associated with shorter distance covered during the 6MWT.29
Decreased exercise capacityThe cardiopulmonary exercise test (CPET) may be useful for objectively demonstrating the causative mechanisms in patients with dyspnea after PE. CPET identifies patterns that allow establishing the mechanism limiting exercise tolerance, which after PE may be due to vascular dysfunction, physical deconditioning, or comorbidities. In patients with residual thrombosis, CPET is useful in early detection of those in whom obstruction of the pulmonary vascular bed leads to an abnormal vascular response to exercise and/or ventilatory inefficiency.4,31
Some patients with residual thrombosis have a lower recruitment and distension capacity of the pulmonary vascular bed to adapt to the increase in stroke volume that occurs during exertion. As a result, during exercise, both PAP and PAP/cardiac output ratio increase, a situation that defines pulmonary vascular dysfunction. This increase in right ventricular afterload may cause a decrease in stroke volume and an increase in the compensatory chronotropic response.4,32
At the same time, the presence of vascular occlusions may limit the perfusion of dependent alveolar units and result in ventilation-perfusion mismatch and ventilatory inefficiency, which is most evident during exercise. This inefficiency is reflected in an increased ventilation and CO2 production (VE/VCO2) slope, decreased end-tidal partial pressure of CO2 (PETCO2) at the anaerobic threshold, and increased physiological dead space (VD/VT).4,31,32
The behavior of patients with CTED during exercise is similar to that of patients with CTEPH,32,33 and differs clearly from that of healthy subjects.31,32 Both patient groups have lower O2 uptake and tolerated workload, ventilatory inefficiency and disturbed gas exchange during exercise.31,32 Functional impairment of the right ventricle is greater in CTEPH compared to CTED, which is reflected in a lower O2 pulse (O2 uptake/heart rate) at maximum exercise.32
Likewise, CPET can show that symptoms are not due to ventilatory limitation, secondary to respiratory disease, or due to physical deconditioning.31,34
Hemodynamic changes after pulmonary embolismNon-invasive measurementsSystematic echocardiography performed after a PE has been used to screen for CTEPH, although when interpreting results it is important to bear in mind that this test is not error-free because it is operator-dependent and provides only an indirect estimate of the sPAP. In the classic studio by Ribeiro et al.,35 only 56% of patients had normal sPAP (<30 mmHg) 1 year after the PE. In a study conducted in 744 consecutive patients, it was found that 36 months after the PE, 67.7% of patients had estimated sPAP values below 36 mmHg, considered of low probability for pulmonary hypertension (PH).36 The estimated incidence of sPAP >50 mmHg was 8.3%.36 Similar figures were found in an analysis of the Computerized Registry of Patients with Thromboembolic Disease (RIETE): 11.1% of patients had estimated sPAP >50 mmHg.37 In this study, patients with elevated sPAP were predominantly women over 70 years, with a history of heart failure or chronic obstructive pulmonary disease, renal failure, and varicose veins,37 so it is possible that, in some patients, this increase in sPAP was due to the observed comorbidities. Other prospective studies in which systematic echocardiography was performed show that only a small percentage of patients have an abnormal echocardiogram during follow-up after acute PE.38 In an analysis performed in 2256 patients with PE from the RIETE registry, in which patients with a history of cardiac or pulmonary disease were excluded, it was found that 6–12 months after the PE, 26% of patients had echocardiographic findings of moderate or high risk for PH.39 This figure clearly overestimates the incidence of CTEPH after PE, and calls into question systematic echocardiography as an early detection tool for CTEPH.38 There is no information to date on the possible role of exercise echocardiography in the detection of exercise PH in patients with post-PE sequelae.
Invasive measurementsNo studies have systematically assessed pulmonary hemodynamics using right heart catheterization (RHC) after acute PE, given the invasive nature of this test. Hemodynamic assessment has usually been limited to cases in which the echocardiogram has shown findings suggestive of PH.
A recent meta-analysis of the incidence of CTEPH in patients followed up for 2 years after an episode of PE shows a prevalence of PH of 0.6%, diagnosed by RHC, among all patients who had PE.23 However, the prevalence was significantly higher among post-PE survivors: 3.2%. Moreover, if only survivors without major comorbidities were considered, the prevalence was 2.8%.23
The prevalence of patients with abnormal mean PAP (mPAP) values (>20 mmHg)—consistent with the new definition of PH proposed at the 6th World Symposium on Pulmonary Hypertension (WSPH)40—or those who present exercise PH after PE is unknown.
In a study conducted in 14 patients with persistent thrombosis, without PH at rest, Van Kan et al.4 demonstrated that these patients had abnormal pulmonary hemodynamic behavior during exercise, with increased ventilatory inefficiency, increased mPAP/cardiac output ratio, and decreased pulmonary vascular distensibility,4 findings that are consistent with the concept of vascular dysfunction.
Chronic thromboembolic pulmonary hypertensionCTEPH is the most serious complication within the spectrum of post-PE complications. It is defined by the presence of PH associated with pulmonary perfusion defects after at least 3 months of effective anticoagulation.40 Among patients with CTEPH, a significant percentage (about 25%41) have no history of an acute PE episode. The pathogenetic mechanisms of CTEPH are not well known. They involve, on the one hand, the thrombotic lesions themselves as a reflection of the failure of endogenous thrombolysis,42,43 and on the other, changes in the pulmonary microvasculature of non-occluded areas, where remodeling and vascular dysfunction occur. These changes are similar to those observed in pulmonary arterial hypertension and are attributed to the increased blood flow resulting from the “diverting” shift of pulmonary flow from occluded to non-occluded areas.2,42
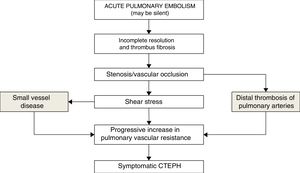
It is not known why only a small proportion of patients who have had PE develop CTEPH. Prothrombotic factors such as elevated factor VIII levels and antiphospholipid antibodies have been associated with the development of CTEPH.44,45 Factors related to angiogenesis have also been implicated, such as vascular endothelial growth factor and the Notch signaling pathway.46 Involvement of the bronchial circulation in the development of CTEPH has recently been suggested, based on the demonstration of anastomosis between bronchial arteries and precapillary arterioles or pulmonary veins. The pressure gradient between the bronchial (systemic) and pulmonary circulation can lead to changes in the pulmonary microvasculature with increased muscle fibers and fibrotic wall thickening47 (Fig. 2).
The latest clinical guidelines on the diagnosis and treatment of PE from the European Society of Cardiology (ESC)-European Respiratory Society (ERS) include the following as risk factors for the development of CTEPH after PE: a) findings relative to the acute PE event, including previous episode of venous thrombosis, large thrombus in the imaging assessment, presence of RV dysfunction in the echocardiogram, and CT angiography findings suggestive of chronic disease19; and b) predisposing concomitant factors, including ventricular-atrial shunts, chronic catheter infection, pacemaker implantation, splenectomy, hypercoagulability states, non-O Rh− blood group, hypothyroidism treated with thyroid hormones, history of cancer, myeloproliferative disorders, inflammatory bowel disease, and chronic osteomyelitis.19
CTEPH should always be ruled out as a cause of PH, so V/Q screening is recommended in all cases of PH.3 If abnormal, diagnostic evaluation of patients with CTEPH includes RHC to characterize the hemodynamic status and imaging tests to identify the location of the lesions (selective digital subtraction pulmonary angiography and/or CT angiography).3,48
The treatment of choice for CTEPH is pulmonary endarterectomy (PEA), so all patients with CTEPH should be evaluated by a multidisciplinary committee in a center with experience in this surgical procedure.48 In patients not requiring PEA, pharmacological treatment with specific drugs for pulmonary arterial hypertension is indicated. In some cases, balloon pulmonary angioplasty may be considered in specialized centers with experience in the procedure.3,48
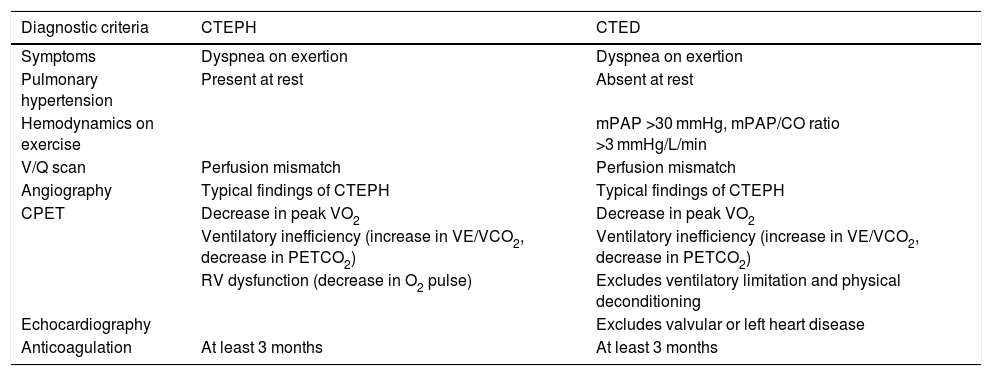
Chronic thromboembolic diseaseCTED is characterized by symptoms, exercise limitation, and residual thrombosis after PE, similar to those of CTEPH, but without PH at rest.3 This entity has received other names such as post-thrombotic pulmonary syndrome3 or chronic thromboembolic pulmonary vascular disease.3,49 We prefer pulmonary chronic thromboembolic disease, as proposed in the 6th WSPH (Table 3)3 and the latest clinical guidelines on PE from the ESC-ERS.19 The concept of CTED arose when PH was defined by mPAP ≥25 mmHg. The 6th WSPH’s current proposal to reduce the threshold for defining PH to mPAP >20 mmHg (with pulmonary vascular resistance >3 Wood units)3 means that patients previously diagnosed with CTED could now be diagnosed with CTEPH.
Differences between chronic thromboembolic pulmonary hypertension and chronic thromboembolic pulmonary disease.
| Diagnostic criteria | CTEPH | CTED |
|---|---|---|
| Symptoms | Dyspnea on exertion | Dyspnea on exertion |
| Pulmonary hypertension | Present at rest | Absent at rest |
| Hemodynamics on exercise | mPAP >30 mmHg, mPAP/CO ratio >3 mmHg/L/min | |
| V/Q scan | Perfusion mismatch | Perfusion mismatch |
| Angiography | Typical findings of CTEPH | Typical findings of CTEPH |
| CPET | Decrease in peak VO2 | Decrease in peak VO2 |
| Ventilatory inefficiency (increase in VE/VCO2, decrease in PETCO2) | Ventilatory inefficiency (increase in VE/VCO2, decrease in PETCO2) | |
| RV dysfunction (decrease in O2 pulse) | Excludes ventilatory limitation and physical deconditioning | |
| Echocardiography | Excludes valvular or left heart disease | |
| Anticoagulation | At least 3 months | At least 3 months |
CO: cardiac output; CPET: cardiopulmonary exercise test; CTED: chronic thromboembolic disease; CTEPH: chronic thromboembolic pulmonary hypertension; mPAP: mean pulmonary arterial pressure; peak VO2: peak oxygen uptake; PETCO2: end-tidal partial pressure of carbon dioxide; VE: ventilation; VE/CO2: ventilatory equivalent for carbon dioxide; VCO2: carbon dioxide production; V/Q scan: ventilation-perfusion scan.
Adapted from: Kim et al.3.
The issue of whether CTED represents a pre-HPTEC state or involves patients with persistent pulmonary thrombosis in whom the mechanisms leading to the development of CTEPH are not triggered, are currently under discussion. In any case, patients with CTED have symptoms and limited exercise tolerance attributable to pulmonary vascular dysfunction. This vascular dysfunction is evident during exercise, so diagnostic tests performed at rest are often within normal limits, as they lack enough sensitivity to detect pulmonary vasculopathy. However, behavior during exercise in patients with CTED resembles that of patients with CTEPH.31,45
Accordingly, CTED is the condition that occurs as a result of the persistence of thrombi in the pulmonary vascular bed that leads to pulmonary vasculopathy, which limits the distensibility of the pulmonary vascular bed and results in ventilatory inefficiency during exercise. Detection of CTED should therefore be focused on highlighting the persistence of thrombi and the involvement of pulmonary circulation on exercise limitation. On the one hand, the presence of residual thrombosis must be shown, for which a V/Q scan is the most sensitive test, as stated in the 6th WSPH proceedings,3 and on the other hand, pulmonary vascular dysfunction must be confirmed, which can be detected by CPET. This demonstrates a pattern of cardiovascular limitation of exercise tolerance (decreased peak O2 uptake, early anaerobic threshold, reduced O2 pulse), accompanied by signs of ventilatory inefficiency due to vascular obliteration (elevated VE/VCO2 slope, and increased CO2 equivalent and decreased PETCO2 at the anaerobic threshold), ruling out a pattern of ventilatory limitation.
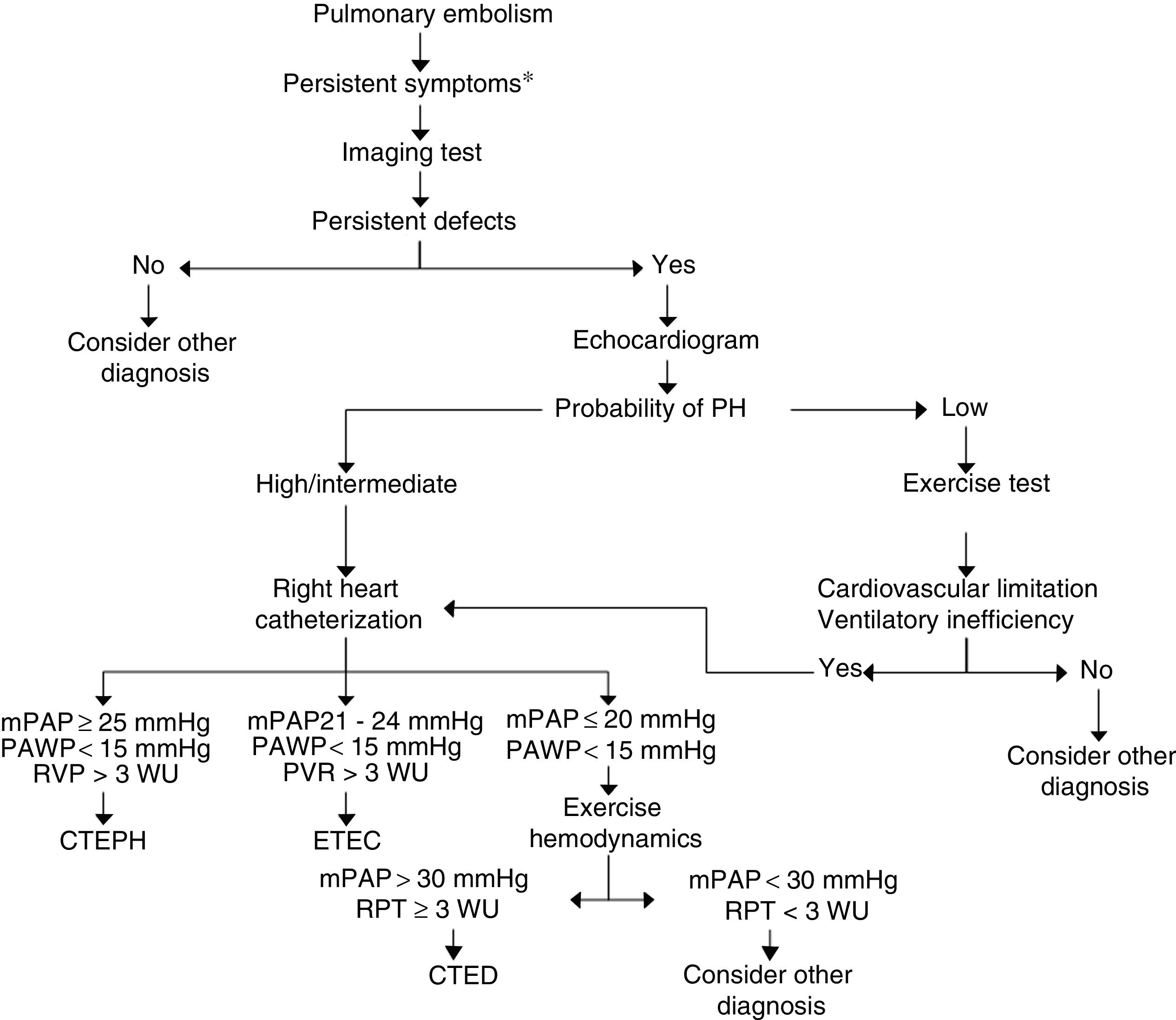
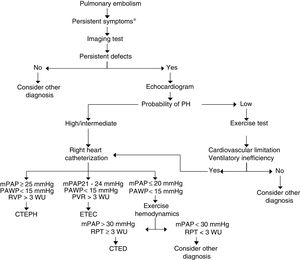
There are still no criteria in the literature for the diagnosis of CTED and its detection after PE. In Fig. 3, we have proposed a PE follow-up algorithm to identify CTEPH or CTED. In symptomatic patients with residual thrombosis, preferably detected by V/Q scan, an echocardiogram should be performed to provide information on the probability of PH. If the probability is high, right heart catheterization should be performed; if low, CPET should be carried out. If the latter reveals a pattern of limited cardiovascular exercise tolerance with criteria for ventilatory inefficiency, it is advisable to perform right heart catheterization, with or without measurements during exercise.50 The diagnosis of CTED should be established in cases with mPAP of 21−24 mmHg at rest, or in those with exercise PH (mPAP >30 mmHg and total pulmonary resistance >3 WU).
Diagnostic algorithm for chronic thromboembolic disease. *Persistent symptoms: persistence of dyspnea after pulmonary embolism, increased dyspnea and/or onset of limited exercise tolerance with no other causes to explain it.
CTED: chronic thromboembolic disease; CTEPH: chronic thromboembolic pulmonary hypertension; mPAP: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; TPR: total pulmonary resistance; WU: Wood units (mmHg/L/min).
In recent years, small case series have been published of patients with symptomatic chronic pulmonary thromboembolism, but without PH (which corresponds to the clinical profile of CTED), in whom PEA was performed.51,52 Donahoe et al.52 included a series of 179 patients who had undergone PEA, 22 of whom had CTED with mPAP of 21 mmHg and pulmonary vascular resistance of 252 dynes s cm−5. Patients were followed up for a median of 23 months after the intervention. After surgery, mPAP fell to 17 mmHg and the functional class improved. Complications in CTED were less frequent and less severe than in CTEPH.1 Among patients with CTED in whom PEA was not performed, one progressed to CTEPH.
Taboada et al.51 described 20 patients with a similar profile: chronic pulmonary thromboembolism, mPAP <25 mmHg at rest, functional limitation and exertional symptoms, and increased dead space measured by CPET. After surgery, patients showed improvement in symptoms, functional capacity, and quality of life questionnaire scores, comparable to CTEPH patients.
PEA in patients with CTED and exercise PH significantly improves functional class and exercise tolerance with improved ventilatory efficiency.4
Studies on the treatment of CTED with specific pulmonary arterial hypertension drugs are not yet available. A small series of 10 patients with CTED treated with balloon pulmonary angioplasty has recently been published, in whom clinical and hemodynamic improvement was observed.53
In any case, in the absence of further evidence regarding the treatment of this entity and the standardization of diagnostic procedures during exercise, it is recommended that assessment of these patients, as in CTEPH, be performed at reference centers with experience in the surgical treatment of CTEPH.
Despite the potential positive effects of cardiopulmonary rehabilitation in patients with CTED,54 there is still no evidence to generate recommendations.
ConclusionPersistent thrombotic lesions in patients with PE are common. The clinical spectrum associated with these lesions is broad, ranging from an asymptomatic course with complete functional recovery to CTEPH. The concept of CTED has emerged in recent years to describe a subgroup of patients with persistent thrombotic lesions who have symptoms on exertion and pulmonary vascular dysfunction, but no PH at rest. The prevalence of this entity is unknown and the criteria for diagnosing it are not clearly defined. Most experts agree that demonstration of persistent thrombotic lesions, development or worsening of symptoms after PE and the demonstration of cardiovascular exercise intolerance, together with normal pulmonary hemodynamics at rest, indicate a diagnosis of CTED. Based on small series of CTED cases that have benefited from PEA, patients with CTED should be assessed in centers with experience in CTEPH and PEA in order to consider the potential indication for such surgery, or the performance of therapeutic procedures analogous to those used in CTEPH, whose risk/benefit ratio should be analyzed individually. In any case, close follow-up of these patients is recommended, as progression to CTEPH cannot be ruled out.
FundingFunded by: Instituto de Salud Carlos III (Spain) (Spain): grants PI15/00582 and PI15/01085.
Conflicts of interestPRM: reports having received subsidies for attendance at conferences and scientific meetings from Actelion, Bayer Healthcare and MSD. She has participated in advisory committees for Actelion and Bayer, and has received support for training from Glaxo Smithkline and Actelion.
ROC: reports having received subsidies for attendance at conferences and scientific meetings from Sanofi, Leo-Pharma, Rovi, Glaxo Smithkline, Actelion, Bayer Healthcare and MSD. She has participated in advisory committees for Actelion, Bayer, Rovi and Leo-Pharma and has received support for research projects from Leo-Pharma and Bayer Healthcare.
JAB: reports having received honoraria for conferences and participation in advisory committees from Actelion, Arena Pharmaceuticals, GSK and MSD; and research grants through his institution from Actelion, GSK, MSD and Ferrer.
Please cite this article as: Ramírez P, Otero R, Barberà JA. Enfermedad tromboembólica crónica pulmonar. Arch Bronconeumol. 2020;56:314–321.