In the scientific literature, contradictory results have been published on the prognostic value of the loss of expression of blood group antigen A (BAA) in lung cancer. The objective of our study was to analyze this fact in our surgical series.
Patients and methodsIn a multicenter study, 402 non-small-cell lung cancer (NSCLC) patients were included. All were classified as stage-I according to the last 2009-TNM classification. We analyzed the prognostic influence of the loss of expression of BAA in the 209 patients expressing blood group A or AB.
ResultsThe 5-year cumulative survival was 73% for patients expressing BAA vs 53% for patients with loss of expression (P=.03). When patients were grouped into stages IA and IB, statistical significance was only observed in stage I-A (P=.038). When we analyzed the survival according to histologic type, those patients with adenocarcinoma and loss of expression of BAA had a lower survival rate that was statistically very significant (P=.003). The multivariate analysis showed that age, gender and expression of BAA were independent prognostic factors.
ConclusionsThe loss of expression of blood group antigen A has a negative prognostic impact in stage I NSCLC, especially in patients with adenocarcinoma.
En la literatura científica se han publicado resultados contradictorios sobre el valor pronóstico de la pérdida de la expresión del antígeno de grupo sanguíneo A (GSA) en el cáncer de pulmón, por lo que analizamos retrospectivamente este hecho en nuestra serie quirúrgica.
Pacientes y métodosEn un estudio multicéntrico de 402 pacientes con carcinoma no microcítico de pulmón (CNMP) en estadio I patológico según la nueva clasificación TNM-2009 se analizó la influencia pronóstica de la pérdida de la expresión del antígeno del GSA en los 209 pacientes con grupos sanguíneos A o AB.
ResultadosLa supervivencia a los 5 años de los pacientes en estadio I patológico que mantenían la expresión del antígeno del GSA fue del 73%, frente a una supervivencia del 53% en los pacientes que habían perdido la expresión del mismo (p=0,03). Cuando se analizó la supervivencia subdividiendo la muestra en estadios IA y IB, solo se alcanzó la significación estadística en el estadio IA (p=0,038). Al analizar la supervivencia según el tipo histológico, los pacientes con adenocarcinoma que perdían la expresión del antígeno del GSA tenían una menor supervivencia, con una p estadísticamente muy significativa (p=0,003). El análisis multivariable mostró que la edad, el género y la expresión del antígeno del GSA eran factores pronósticos independientes.
ConclusionesLa pérdida de la expresión del antígeno del grupo sanguíneo A tiene una influencia pronóstica negativa en el CNMP estadio I patológico, sobre todo en el tipo histológico adenocarcinoma.
Lung cancer is the first cause of mortality due to neoplastic disease,1 and its prognosis is still poor despite the advances made in medicine in recent decades.2
In 2009, the new TNM classification for lung cancer was published,3,4 which has introduced important modifications in the staging system that have been extensively validated.5
Approximately 80% of all lung cancers belong to the group of non-small-cell lung cancer (NSCLC). The most important prognostic factor in NSCLC is tumor staging. Factors that worsen prognosis are male sex and advanced age.6 Complete surgical resection is the treatment of choice in the initial stages of NSCLC, although the surgical approaches and methods are evolving.7 Of those patients with NSCLC in stage I who undergo complete resection approximately 30% relapse and do not survive the following 5 years. This is basically due to the presence of micrometastatic disease at the time of resection,8 although the mechanisms involved are not understood and we do not know in which patients these relapses will occur.
ABH blood group antigens were originally described in the membrane of the red blood cells, but they have been later found in the majority of the epithelia, endothelia and secretions of the organism.9 They are a variety of glycolipids and glycoproteins whose antigenic specificity is determined by the variation in the constituents of their carbohydrate chains.10 For several decades, we have known about the existence in tumors of changes in the glycosylation patterns of the glycolipids and glycoproteins of the cell surface. Recent studies appear to indicate that the said alterations are the result of an initial oncogenic transformation and a key element in the induction of tumor invasions and in the development of metastasis.11 In some tumors, the high expression of certain glycosylated antigens promotes invasion and metastasis, while the expression of other glycosylated antigens suppresses tumor progression. The expression mechanism of these antigens has been widely studied with regards to the state of the genes of their respective glycosyltransferases. However, we know very little about the mechanisms through which the glycosylated antigens induce invasive and metastatic phenotypes in the tumor cells.12 The antigenic determinants of the ABH blood group are located in the terminal portion of the carbohydrate chains of the glycolipids and glycoproteins of the cell membrane. Their degree of expression depends on the type of differentiation of the epithelium and the degree of maturation of the cell within it.13
The loss of blood antigen A expression is caused by the loss of specific glycosyltransferase activity secondary to the deletion or the loss of heterozygosity in the long arm of chromosome 9 (one of the chromosomes more intensely involved in carcinogenesis) where their alleles are located, or to DNA hypermethylation of their promoter region, which impedes transcription.14 The loss of the expression of the ABH blood group antigens was described for the first time in gastric cancer.15 Since then, this phenomenon and its relationship with prognosis have been described in carcinomas of the head and neck, lungs, gastrointestinal tract, ovaries, bladder and breast.14 In the case of lung cancer, a very few articles have been published in this regard. Specifically, the first to relate lung cancer prognosis with the loss of blood antigen A (BAA) expression was that by Lee et al.16 However, in other articles this prognostic relationship has not been able to be demonstrated.17,18
Due to the controversy in the literature, the objective of this study has been to carry out an extensive multicenter study in order to analyze the prognostic influence of the loss of BAA antigen expression in stage I NSCLC according to the new TNM-2009 staging system.
Patients and methodsPatientsOur surgical series, which was the object of a previous study about survival and prognostic factors published in this journal in September, 2011,19 was made up of patients who underwent surgery due to NSCLC at the Thoracic Surgery Departments of the Hospital General Universitario “Gregorio Marañón” in Madrid (between 1991 and 2005), the Complejo Hospitalario Xeral-Cies in Vigo (between 2000 and 2004) and the Complejo Hospitalario Universitario in Albacete (between 2001 and 2006). The patients were in pathologic stage I and the intervention involved complete lung resection, with no adjuvant or induction treatment. The resection was considered complete when the surgical edges were tumor-free and there was no metastasis in the lymph nodes analyzed after systematic mediastinal lymphadenectomy. We excluded those patients who died within 30 days after surgery and those who had no post-operative follow-up. The following variables were collected: age, sex, type of resection, histologic type, degree of differentiation, tumor size and stage (IA and IB) according to the new 2009 TNM classification for lung cancer.3 In the post-op follow-up in the outpatient consultation, relapses and causes of death were recorded. It was considered that there was a loco-regional or distant relapse when the evidence of the clinical, analytic, radiologic (basically compatible findings on CT) or histologic criteria or a combination of these supported the existence of said secondary affectation. Due to the fact that this is a retrospective study of patients who underwent surgery for lung cancer, they all signed an informed consent document prior to surgery which authorizes the use of their data for research. It was therefore not required by the ethics committee for a new specific informed consent form for this study to be signed.
ImmunohistochemistryThe tumor blocks in paraffin from the patients of the Complejo Hospitalario Xeral-Cies in Vigo and the Complejo Hospitalario Universitario in Albacete were sent to the Pathology Department of the Hospital General Universitario Gregorio Marañón, where the histologic samples were analyzed by two pathologists to confirm the histologic type and immunohistochemistry was determined. The current lung tumor classification of the WHO from 2004 was used.20
After carrying out the immunohistochemistry study that is detailed below, the patients who expressed the BAA in normal bronchopulmonary tissue were identified as belonging to blood groups A or AB. In this patient subgroup, the prognostic value of the loss of BAA expression was studied.
The antibody used was BG-2 (clone T36), which is valid for tissues included in paraffin and corresponds with an IgG3 type that is specific for the Type 1 and 2 chains of BAA (GeneTex Inc., Irvine, CA, USA).
The immunostaining technique used was the ABC system (avidin–biotin complex) with the following steps: dewax the sections with xylene, ethanol and 96° alcohol; hydration; block endogenous peroxidase by immersion in a solution of oxygenated water in 5% methanol for 5min; wash in PBS solution; incubate with blocking serum; incubate with the primary antibody; wash in PBS; incubate with the biotinated secondary antibody; incubate with the avidin–peroxidase–biotin complex; wash in PBS; incubate with the diaminobenzidine chromogen solution; wash in distilled water; light contrast stain with Carazzi's hematoxylin; wash briefly with running water, dehydration, rinse and mount on permanent medium.
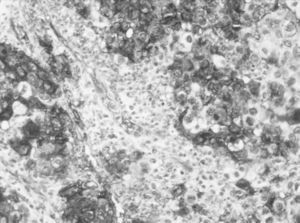
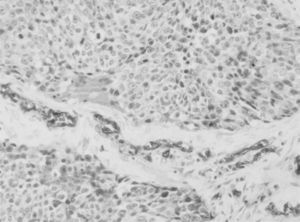
As in the great majority of the articles published about the prognostic value of the loss of tumor expression in lung cancer,16–18,21,22 in our study the expression in the tumor tissue of blood group A antigen in patients with blood types A or AB was considered positive when the percentage of stained cells was greater than 5%. The result was considered negative when the cells demonstrated not staining compared with internal controls (bronchial epithelium, bronchial glands, vascular endothelium and erythrocytes). When the cells stained between 1% and 5%, the result was considered equivocal and they were grouped with the negative cases for the statistical analysis (Figs. 1 and 2).
The statistical analysis was done using SPSS 15.0 software for Windows. The normality of the quantitative variables was demonstrated with the Kolmogorov–Smirnov test. The comparison of the independent quantitative variables was done: if the variable followed a normal distribution using the Student's t test, and if the variable followed a non-normal distribution with the Mann–Whitney U or the Kruskal–Wallis tests. The relationship between two categorical variables was studied with the chi-squared test. The survival analysis was carried out by means of the Kaplan–Meier method and to compare the survival curves the log-rank test was used. For the multivariate analysis, we constructed a Cox proportional hazards multiple regression model (“enter” method). In this way, we calculated the risk of death for each variable, controlling for the presence or absence of the rest of the variables included in the model. A P value less than .05 was considered statistically significant.
ResultsThe sample selected for the study was made up of 402 patients,19 of whom 180 underwent interventions at the Hospital General Universitario “Gregorio Marañón” in Madrid, 135 at the Complejo Hospitalario Xeral-Cies in Vigo and 87 at the Complejo Hospitalario Universitario in Albacete. Immunohistochemistry showed that 209 patients (52%) expressed BAA in normal bronchopulmonary tissue and were therefore identified as belonging to the A or AB blood groups.
Out of the 209 patients with blood groups A or AB, 126 (60%) maintained the expression of the blood group A antigen in the tumor and 83 (40%) had lost it. 182 (87%) were males and 58 (28%) were over the age of 70. The most frequent resection was lobectomy (70%). The distribution by histologic type, degree of differentiation and stages are shown in Table 1.
After a mean follow-up of 70.18 months (range: 1.8–218.07), 98 patients (47%) were alive and disease-free, 8 patients (4%) were alive with tumor relapse, 71 patients (34%) died due to tumor relapse and 32 patients (15%) died due to other causes.
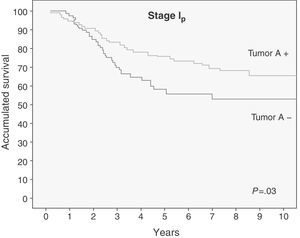
Median survival was not reached. Five-year survival of the patients with blood groups A or AB and non-small-cell lung cancer in pathologic stage I that maintained BAA expression in the tumor was 73%, compared to a survival of 53% of the patients who had lost the expression. The difference found was statistically significant, as shown in Fig. 3.
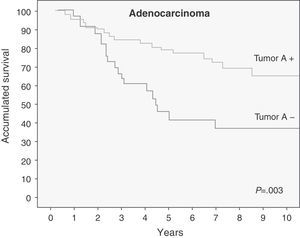
Survival was also analyzed depending on the tumor expression of BAA, stratified by stages. Both in stage IA as well as in IB, we observed a relevant clinical influence, but this only reached statistical significance in stage IA (Table 2). Last of all, we analyzed survival depending on the tumor expression of BAA, stratifying according to histologic type (Table 2). The patients with adenocarcinoma who had lost BAA expression had a shorter survival (Fig. 4), with a P that was statistically very significant (P=.003). This difference was not found in the epidermoid carcinoma (P=.58). This relationship was not studied in large-cell carcinoma due to the limited number of patients in this group (7 patients).
Five-year Survival Results of the Patients With Blood Groups A or AB, According to BAA Expression, Stratifying According to Stage and Histologic Type.
| Expression of the Antigen del BAA | |||
| Negativen–Sv% | Positiven–Sv% | P | |
| Total 5-year survival (Sv%) | |||
| Stage | |||
| IA | 31%–63% | 46%–86% | .038 |
| IB | 52%–51% | 80%–60% | .20 |
| Histologic type | |||
| Epidermoid | 45%–65% | 55%–67% | .58 |
| Adenocarcinoma | 36%–36% | 66%–65% | .003 |
n: number of patients; Sv%: total 5-year survival in percentage; BAA: blood antigen A.
In the multivariate analysis done with the Cox regression, we included the same variables as in our previous study about prognostic factors in stage I NSCLC19: age (>70 vs ≤70), sex, histologic type, type of resection and pathologic stage (IB vs IA), together with the variable “expression of blood group A antigen”. The multivariate analysis showed that age, sex and BAA expression were independent prognostic factors (Table 3).
Multivariate Analysis According to the Cox Proportional Hazards Model of the Patients With Blood Types A or AB.
| Multivariate Survival Analysis | |||
| P | HR | 95% CI of HR | |
| Age (>70 vs ≤70) | 0.035 | 1.033 | 1.002–1.065 |
| Sex (male vs female) | 0.040 | 2.936 | 1.052–8.190 |
| Histologic type | 0.184 | – | – |
| *Epidermoid vs adenocarcinoma | 0.071 | 1.596 | 0.961–2.650 |
| *Epidermoid vs large-cell carcinoma | 0.987 | 1.012 | 0.238–4.291 |
| Type of resection | 0.799 | – | – |
| *Atypical resection vs lobectomy | 0.795 | 1.092 | 0.564–2.111 |
| *Atypical resection vs pneumonectomy | 0.517 | 1.354 | 0.541–3.384 |
| Stage (IB vs IA) | 0.068 | 1.657 | 0.964–2.849 |
| BAA expression (no vs yes) | 0.045 | 1.628 | 1.010–2.626 |
HR: hazards ratio; vs: versus; CI: confidence interval; BAA: blood antigen A.
In our series of 209 patients with blood types A or AB and non-small-cell lung cancer in stage I (according to the 7th edition of the TNM classification from 2009), we have demonstrated that the loss of blood antigen A expression is an unfavorable prognostic factor (P=.03), fundamentally in the adenocarcinoma histologic type (P=.003).
The first group of researchers to propose this prognostic association were Lee et al.16 in 1991, in a study of 164 patients. However, in their study there were only 27 patients in stage I with blood types A or AB.
In 1997, Graziano et al.23 studied a series of 260 patients, of whom 70 were stage I. They found that the 29 patients who had lost BAA expression had an average survival of 41 months, compared with 41 patients who maintained BAA expression and had a median survival of 98 months (P=.01).
Similar results were obtained by Ulger et al.21 in 2002, in their series of 92 patients with lung cancer at any stage and of any histologic type (including small-cell).
In contrast, other articles like that by Gwin et al.22 published in 1994 and the articles by Rice et al.18 and Dresler et al.17 published in 1995 found no relationship between prognosis and the loss of BAA expression.
Matsumoto et al.24 found that the loss of expression of the antigens of blood group ABH in lung cancer correlated with a greater potential for metastasis, fundamentally with the recurrence of distant hematogenous metastases. Ichikawa et al.,25,26 in experimental studies with cell lines of human colon carcinoma, found a more limited mobility of the cells that express said antigens (fundamentally antigen A), which could explain the higher metastatic capacity of the cells that have lost their expression.
Our study has centered on determining the prognostic value of the loss of BAA expression in patients with NSCLC in pathologic stage I due to the fact that in a study to determine prognostic factors, the initial stages are those that are least influenced by other elements whose impact on survival is widely known (lymph node affectation, distant metastasis, etc.). In addition, the patients have been re-staged according to the new TNM classification for 2009 by the UICC and AJCC,3,4 which is more restrictive in the definition of stage I as patients with tumors larger than 5cm are excluded. On the other hand, we have only included patients with NSCLC due to the fact that small-cell carcinoma has a very different biological behavior and prognosis. Lastly, we excluded patients who received either adjuvant or induction treatment in order to avoid the influence on survival that complementary treatment entails, which could add a confounding factor to our results and conclusions.
Having done a retrospective, multicenter study with long data-collection periods, which were different among the 3 participating hospitals and from which we have excluded the lost cases, could to a certain measure limit the validity of our results if we compared them with those from a hypothetic prospective and randomized study. Nevertheless, the strict selection criteria of the sample described previously have allowed us to identify a homogenous group of patients with limited variability in the main clinical and anatomic prognostic factors. In this way, the analysis of the prognostic value of the loss of blood group A antigen expression in non-small-cell carcinomas in pathologic stage I have not been altered by other factors with known influence on survival. On the other hand, having carried out a multicenter study has offered us the means to include the most extensive series of patients with the longest mean follow-up (>70 months) ever published in the literature about the prognostic value of the loss of BAA expression in lung cancer, which gives a strong consistency to our statistical results.
When we analyzed the 5-year survival depending on the loss of the tumor expression of BAA, stratifying according to stage, statistical significance was only reached in the case of stage IA (P=.038), probably due to the successive subdivisions of the sample.
The most relevant fact was found when analyzing 5-year survival according to tumor expression of BAA, stratified by histologic type, as the patients with adenocarcinoma who lost BAA expression had a poorer prognosis, with a statistically very significant P value (P=.003). After having done a bibliographic search in Pubmed, we found that this fact had only been reported by Moldvay et al.27 These authors, in their study on prognostic factors of surgically resected NSCLC, found that in the adenocarcinomas there were frequent deletions of chromosome 9, where alleles A and B are located, which cause the accumulation of their fucosylated precursors which function as presenters of ligands to adhesion receptors such as E-Selectin. All these promote the capacity of the tumor cells to interact with activated platelets, leucocytes and endothelial cells and, therefore, facilitate metastatic dissemination.
The multivariate analysis showed that the loss of tumor expression of blood antigen A is an unfavorable prognostic factor. As with other authors6 and the results recently published by our group in this journal,19 in the multivariate analysis we also found that male sex and age over 70 were adverse prognostic factors.
In short, our paper is the multicenter study with a large number of patients reported in the literature about the protective function of maintaining BAA expression in pathologic stage I NSCLC, fundamentally in the adenocarcinoma histologic type. This could be related with the smaller metastatic potential of the tumor cells that maintain BAA expression due to motility inhibition, although we still do not know the biomolecular mechanisms that are responsible.
Conflict of InterestThe authors declare having no conflict of interests.
Please cite this article as: León-Atance P, et al. Influencia pronóstica de la pérdida de la expresión del antígeno del grupo sanguíneo A en el carcinoma no microcítico de pulmón en estadio I patológico. Arch. Bronconeumol. 2011:48:49–54.