Immune cell functional assay (ImmuKnow®) is a non-invasive method that measures the state of cellular immunity in immunosuppressed patients. We studied the prognostic value of the assay for predicting non-cytomegalovirus (CMV) infections in lung transplant recipients.
MethodsA multicenter prospective observational study of 92 patients followed up from 6 to 12 months after transplantation was performed. Immune cell functional assay was carried out at 6, 8, 10, and 12 months.
ResultsTwenty-three patients (25%) developed 29 non-CMV infections between 6 and 12 months post-transplant. At 6 months, the immune response was moderate (ATP 225–525ng/mL) in 14 (15.2%) patients and low (ATP<225ng/mL) in 78 (84.8%); no patients had a strong response (ATP≥525ng/mL). Only 1 of 14 (7.1%) patients with a moderate response developed non-CMV infection in the following 6 months compared with 22 of 78 (28.2%) patients with low response, indicating sensitivity of 95.7%, specificity of 18.8%, positive predictive value (PPV) of 28.2%, and negative predictive value (NPV) of 92.9% (AUC 0.64; p=0.043). Similar acute rejection rates were recorded in patients with mean ATP≥225 vs. <225ng/mL during the study period (7.1% vs. 9.1%, p=0.81).
ConclusionAlthough ImmuKnow® does not seem useful to predict non-CMV infection, it could identify patients with a very low risk and help us define a target for an optimal immunosuppression.
El test de función de la inmunidad celular (ImmuKnow®) es un método que mide el estado de la inmunidad celular en pacientes inmunosuprimidos. Se estudió su valor pronóstico para predecir infecciones diferentes a citomegalovirus (CMV) en receptores de un trasplante pulmonar.
MétodosSe realizó un estudio observacional prospectivo multicéntrico de 92 pacientes seguidos desde los 6 a los 12 meses postrasplante. El test se realizó a los 6, 8, 10 y 12 meses.
ResultadosVeintitrés pacientes (25%) desarrollaron 29 infecciones no debidas a CMV entre los 6 y los 12 meses posteriores al trasplante. A los 6 meses, la respuesta inmune fue moderada (ATP 225-525ng/ml) en 14 (15,2%) pacientes y baja (ATP<225ng/ml) en 78 (84,8%); ningún paciente tuvo una respuesta fuerte (ATP>525ng/ml). Solo uno de 14 (7,1%) pacientes con una respuesta moderada desarrolló una infección diferente a CMV en los 6 meses siguientes a la realización del test en comparación con 22 de 78 (28,2%) con respuesta baja, indicando una sensibilidad del 95,7%, una especificidad del 18,8%, un valor predictivo positivo del 28,2% y un valor predictivo negativo del 92,9% (AUC 0,64; p=0,043). Se registraron tasas de rechazo agudo similares en pacientes con ATP medio >225 frente a <225ng/ml durante el período de estudio (7,1 frente al 9,1%; p=0,81).
ConclusiónAunque el test ImmuKnow® no parece útil para predecir infecciones diferentes al CMV, podría identificar pacientes con riesgo muy bajo y ayudarnos a definir un objetivo de inmunosupresión óptima.
Infection is one of the main causes of morbidity and mortality throughout the life of lung transplant recipients. The frequency of infection in lung transplantation is much higher than in other types of solid organ transplant (SOT).1 However, there are no tests that enable us to evaluate the specific risk of infection in a given patient. The immune cell functional assay (ImmuKnow®) is a noninvasive technique used to evaluate the global immune response. It has been approved by the United States Food and Drug Administration for monitoring immunosuppression in solid organ recipients. The assay quantifies production of adenosine triphosphate (ATP) in CD4+T lymphocytes when these are stimulated in vitro with phytohemagglutinin (PHA) and is both reproducible and affordable.2,3 In published studies, its usefulness for predicting infections varies with the type of transplant,4,5 and it could prove useful in lung transplantation.6–10 The objectives of this study were to evaluate the prognostic value of this assay for predicting infection other than cytomegalovirus (CMV) in lung transplant recipients. The predictive value was determined in the short term (the 2 months following testing) and in the medium term (the 6 months following testing).
Material and methodsDesign and study populationThis was a multicenter, prospective, observational study performed at 7 centers with a lung transplant program. The study population comprised 92 transplant recipients who were followed up during the period 6–12 months post-transplant. Follow-up began at 6 months to minimize other factors influencing infection development (e.g. surgery, postoperative complications or prophylaxis used). To be included, patients had to be adults, have positive pre-transplant CMV serology, and no active infection. Active infection was defined as an infection that produced symptoms and/or in which the microorganism actively replicated infecting new cells and tissues. Patients were recruited between January 2014 and April 2015. The assay was carried out during scheduled patient visits at 6, 8, 10, and 12 months after transplant, and results were blind to clinicians. Any type of infection since the previous visit was recorded. Additional variables recorded included blood or laboratory test results, immunosuppression, respiratory function, acute rejection, and other complications. Acute rejection was classified according to the criteria of the International Society for Heart and Lung Transplantation.11,12 These data were recorded prospectively using a purpose-designed electronic case report form. Data were sent electronically and stored in a central server using the “e-Clinical” methodology according to regulation FDA 21 CRF Part 11, which guarantees data confidentiality, safety, and authenticity.
Written informed consent was obtained from all participants. The study was approved by the Clinical Research Ethics Committee of Hospital Universitari Vall d’Hebron, Barcelona, Spain, which was the lead center (EPA(AG)47/2013), and a further two ethics committees. It was also approved by the health authorities of the relevant autonomous communities. The data that support the findings of this study are available from the corresponding author upon reasonable request.
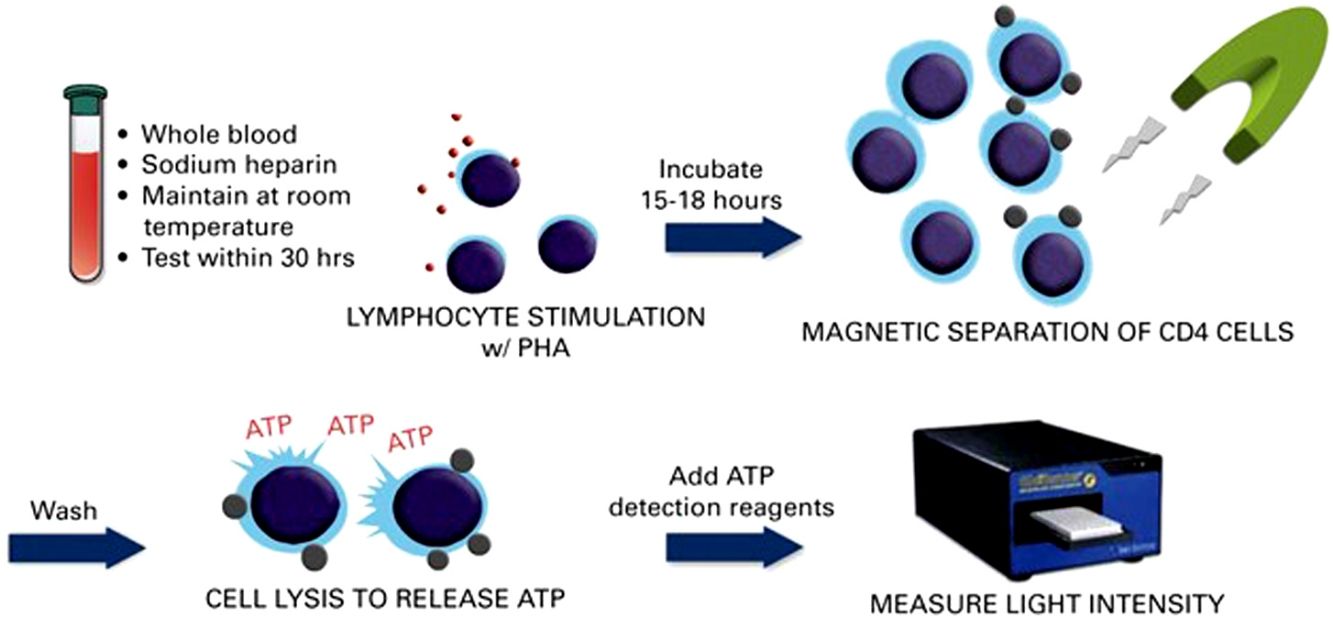
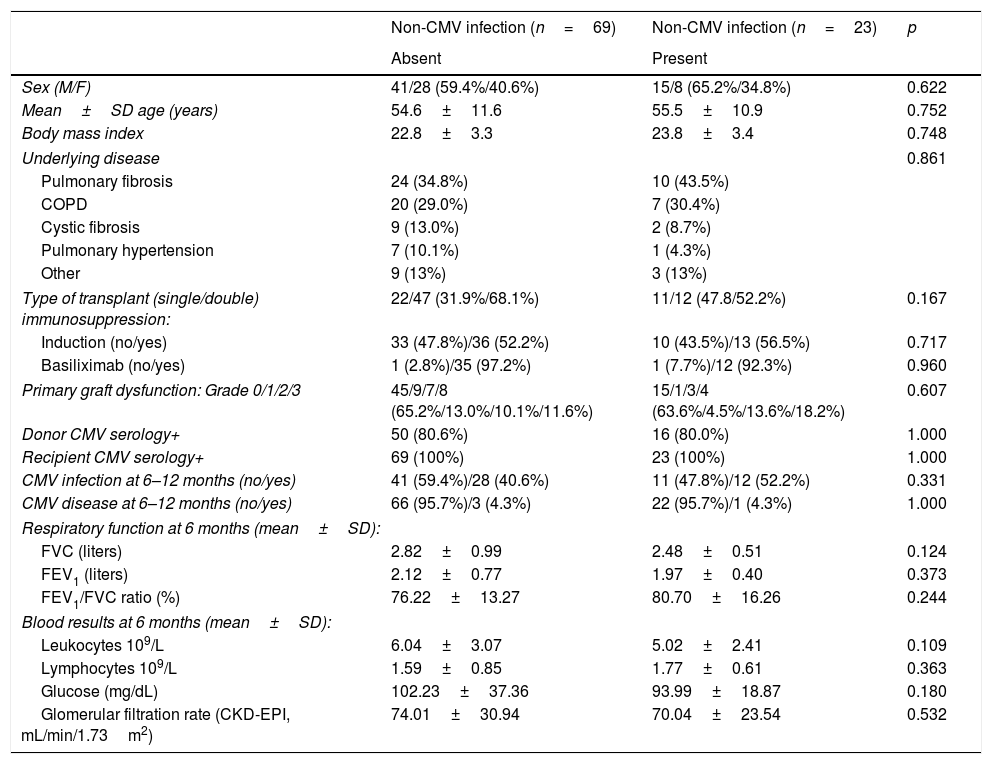
Immune cell functional assay (ImmuKnow®)Patient immune function was assessed using the commercially available ImmuKnow® assay (Cylex Inc., Columbia, USA), which determines intracellular ATP levels in CD4+T cells. Whole blood samples were collected in a sodium heparin vacutainer tube, and the intracellular ATP concentration was measured according to the manufacturer's protocol.
Briefly, 250μL of anticoagulated whole blood was diluted with sample diluent, added to the wells of a 96-well microtiter plate, and incubated for 15–18h with phytohemagglutinin (PHA) in a 37°C, 5% CO2 incubator. Whole blood was incubated concurrently in the absence of stimulant to assess baseline ATP activity. The following day, CD4+T cells were positively selected within the microwells using magnetic particles coated with anti-human CD4 monoclonal antibodies and a strong magnet, washed to remove residual cells, and lysed to release intracellular ATP. Released ATP was detected using luciferin/luciferase mixture and measured in a luminometer at a maximum emission wavelength of 562nm (GloRunner Microplate Luminometer, Turner biosystems, Sunnyvale, CA, USA). ATP concentration (ng/mL) was calculated comparing with a calibration curve. The cut-off values were those recommended by the manufacturer, which were established by testing 155 healthy adults and 127 transplant recipients.13 A low immune response was defined as ATP levels<225ng/mL, moderate as ATP levels 225–524ng/mL, and strong as ATP levels≥525ng/mL. Other cut-off points that could improve the sensitivity and specificity for predicting infection based on receiver operating characteristic (ROC) curves were also investigated.
Antimicrobial prophylaxisIn the immediate postoperative period, patients without preoperative bronchial colonization received amoxicillin-clavulanate, piperacillin tazobactam or imipem according to the protocols of the participating center. In patients with bronchial colonization, antibiotics were modified depending on the latest cultures and inhaled colistin or tobramycin were also given. All patients received CMV prophylaxis, which consisted of intravenous ganciclovir following surgery until oral intake was resumed, then switched to valganciclovir at 900mg/d (dose adjusted to renal function) until 180 days after surgery. Three centers gave isoniazid for 9 months or isoniazid plus rifampicin for 3 months in patients with tuberculosis infection (positive PPD test) before transplantation. Universal prophylaxis for Aspergillus infection was with nebulized amphotericin B (liposomal or lipid complex), and two centers also gave fluconazole; the length of this prophylaxis ranged from 1.5 months to indefinitely, depending on the center. All patients received cotrimoxazole for Pneumocystis jirovecii prophylaxis.
Immunosuppressive regimensPatients were treated according to local protocol with tacrolimus plus mycophenolate mofetil (1–2g/d) or mycophenolic acid (720–1440mg/d) and corticosteroids. Cyclosporine and azathioprine were used in 1 case each. The dose of tacrolimus was adjusted for target trough serum levels of 10–15ng/mL. Methylprednisolone was started in the operating room (10mg/kg) before graft reperfusion, followed by 375mg/d on the first day and gradual tapering over the first year to reach a maintenance dose of 0.1–0.2mg/kg/d. mTOR inhibitors were used as rescue therapy in chronic and recurrent acute rejection or to replace other immunosuppressive agents due to adverse effects. Induction therapy with basiliximab was used according to local protocols. Depending on the severity of the episode, acute rejections were treated with an IV pulse of methylprednisolone 5–10mg/kg/d for 3 days or 1mg/kg/d for 10 days.
Infection definitionsTracheobronchitis was defined as new onset of shortness of breath, cough, sputum, rales, or wheezing plus microbiological isolation from sputum or bronchoscopy sample. When microbiological isolation was not possible, it was considered possible tracheobronchitis if the patient had purulent sputum and responded to antibiotic treatment. Ulcerative or pseudomembranous tracheobronchitis was defined on the basis of observation of necrotic ulcers or pseudomembrane in the anastomosis on by bronchoscopy plus microbiological isolation. Pneumonia was distinguished from tracheobronchitis if it was associated with a new pulmonary infiltrate on chest X-ray or computed tomography. Extrapulmonary infections were defined by microbiological isolation at the site of infection associated with symptoms and signs suggestive of disease. The definitions were adapted from ISHLT consensus for standardization of definitions in cardiothoracic transplant recipients.14
Statistical analysisSample size was determined based on previous reports using the program Ene-3.0.6,7 In order to estimate a proportion with a 2-sided 95% confidence interval and 5% accuracy, and assuming that the expected proportion would be 95%, 73 patients were required. Assuming a 20% loss or invalid results, we aimed to recruit 92 patients to achieve the study objectives.
Student t-test was used to analyze continuous variables with a normal distribution. For those with a non-normal distribution, the Mann-Whitney test (unpaired data) and Wilcoxon test (paired data) were used. The chi-squared test (or Fisher exact test when applicable) was used to analyze contingency tables, proportions, and frequency distribution. The McNemar test was used to measure attributes at 2 different time points. The Pearson or Spearman correlation coefficient was used to determine the correlation between 2 continuous variables. Receiver operating characteristic (ROC) curves were used to determine the sensitivity and specificity of the assay. Positive predictive value (PPV) and negative predictive value (NPV) were calculated using contingency tables. Survival rates were calculated using the Kaplan–Meier method. Statistical comparisons were made using the log-rank test. All hypothesis tests were 2-tailed. Statistical significance was set at p<0.05. Data were analyzed using SPSS version 22.0. Confidence intervals were calculated at 95%.
ResultsBetween months 6 and 12 post-transplantation, 23 of the 92 patients (25.0%) developed 29 non-CMV infections. No significant differences were found between patients who developed infection and those who did not, in terms of the demographic and clinical variables recorded (Table 1).
Demographic and clinical data of patients who developed a non-CMV infection between 6 and 12 months after transplant surgery and those who did not.
| Non-CMV infection (n=69) | Non-CMV infection (n=23) | p | |
|---|---|---|---|
| Absent | Present | ||
| Sex (M/F) | 41/28 (59.4%/40.6%) | 15/8 (65.2%/34.8%) | 0.622 |
| Mean±SD age (years) | 54.6±11.6 | 55.5±10.9 | 0.752 |
| Body mass index | 22.8±3.3 | 23.8±3.4 | 0.748 |
| Underlying disease | 0.861 | ||
| Pulmonary fibrosis | 24 (34.8%) | 10 (43.5%) | |
| COPD | 20 (29.0%) | 7 (30.4%) | |
| Cystic fibrosis | 9 (13.0%) | 2 (8.7%) | |
| Pulmonary hypertension | 7 (10.1%) | 1 (4.3%) | |
| Other | 9 (13%) | 3 (13%) | |
| Type of transplant (single/double) immunosuppression: | 22/47 (31.9%/68.1%) | 11/12 (47.8/52.2%) | 0.167 |
| Induction (no/yes) | 33 (47.8%)/36 (52.2%) | 10 (43.5%)/13 (56.5%) | 0.717 |
| Basiliximab (no/yes) | 1 (2.8%)/35 (97.2%) | 1 (7.7%)/12 (92.3%) | 0.960 |
| Primary graft dysfunction: Grade 0/1/2/3 | 45/9/7/8 (65.2%/13.0%/10.1%/11.6%) | 15/1/3/4 (63.6%/4.5%/13.6%/18.2%) | 0.607 |
| Donor CMV serology+ | 50 (80.6%) | 16 (80.0%) | 1.000 |
| Recipient CMV serology+ | 69 (100%) | 23 (100%) | 1.000 |
| CMV infection at 6–12 months (no/yes) | 41 (59.4%)/28 (40.6%) | 11 (47.8%)/12 (52.2%) | 0.331 |
| CMV disease at 6–12 months (no/yes) | 66 (95.7%)/3 (4.3%) | 22 (95.7%)/1 (4.3%) | 1.000 |
| Respiratory function at 6 months (mean±SD): | |||
| FVC (liters) | 2.82±0.99 | 2.48±0.51 | 0.124 |
| FEV1 (liters) | 2.12±0.77 | 1.97±0.40 | 0.373 |
| FEV1/FVC ratio (%) | 76.22±13.27 | 80.70±16.26 | 0.244 |
| Blood results at 6 months (mean±SD): | |||
| Leukocytes 109/L | 6.04±3.07 | 5.02±2.41 | 0.109 |
| Lymphocytes 109/L | 1.59±0.85 | 1.77±0.61 | 0.363 |
| Glucose (mg/dL) | 102.23±37.36 | 93.99±18.87 | 0.180 |
| Glomerular filtration rate (CKD-EPI, mL/min/1.73m2) | 74.01±30.94 | 70.04±23.54 | 0.532 |
Time (months).
Infections occurred in the form of bacterial or fungal tracheobronchitis in 11 cases (3 cases of Aspergillus spp, 2 cases of Pseudomonas spp and one case each of Staphylococcus aureus, Streptococcus pneumoniae, Morganella spp, Enterococcus spp, Rhizopus spp and Mycobacterium gordonae), 8 cases of possible bacterial tracheobronchitis, and 2 cases of pneumonia (1 Pseudomonas spp and 1 unidentified fungus).
Other diagnoses were 3 cutaneous infections (2 herpes zoster and 1 olecranon bursitis due to Staphylococcus aureus), 2 esophageal candidiasis, 1 gastroenteritis due to Salmonella spp, 1 thoracotomy scar infection with Mycobacterium tuberculosis and 1 endocarditis due to Pseudomonas spp. Three of the 92 patients (3.3%) died before completing the study (1 septic shock secondary to pneumonia, 1 kidney failure, and 1 sudden death).
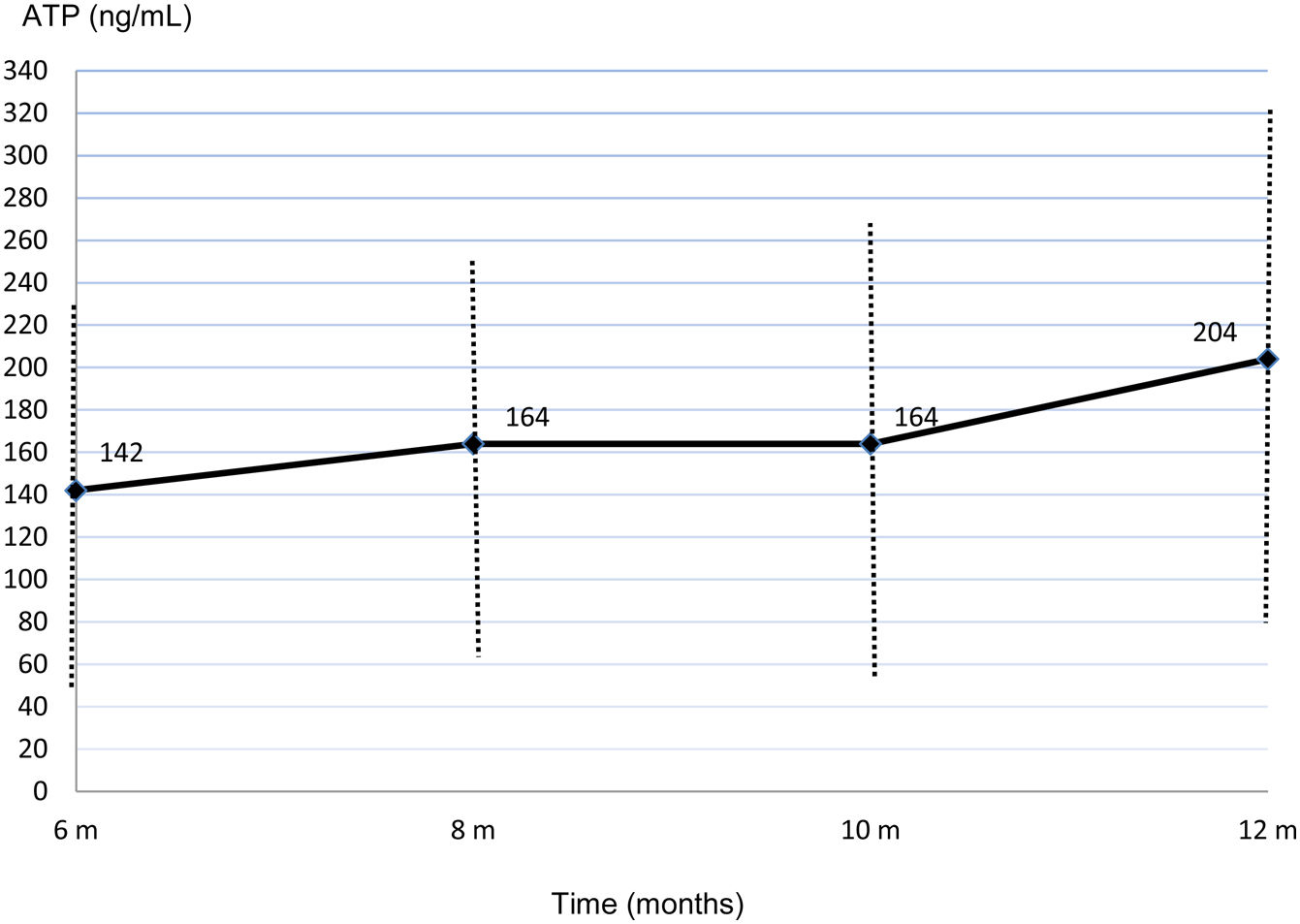
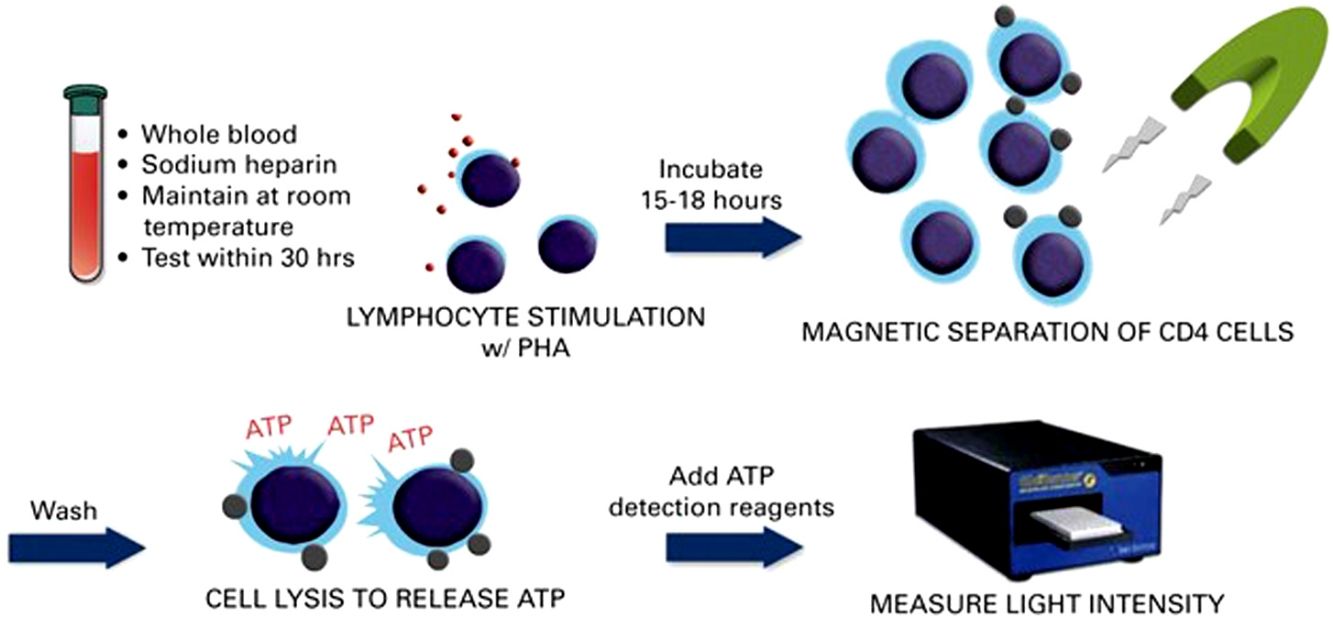
Mean ImmuKnow® values significantly increased from month 6 (142.45±88.60ng/mL) to month 12 (204.44±121.67ng/mL) after surgery (p=0.002) (Fig. 1).
Mean ImmuKnow® values were lower in patients who developed infection than in those who did not (111±58.5ng/mL vs. 155.38±96.40ng/mL, p=0.02). Trough serum tacrolimus levels were similar in both groups (9.70±2.81ng/mL vs. 10.90±3.99ng/mL, p=0.22), as were doses of mycophenolate or mycophenolic acid (1447.36±497.06mg vs. 1366.06±585.53mg, p=0.509) and corticosteroids (12.62±5.12mg vs. 11.07±5.41mg, p=0.164).
We observed a weak correlation between the dose of mycophenolate (mycophenolic acid) and ImmuKnow® values (r=−0.149, p=0.027). No correlation was observed for trough blood levels of tacrolimus (r=−0.069, p=0.28) or dose of corticosteroids (r=−0.037, p=0.560).
Mean ImmuKnow® values between 6 and 12 months post-transplant were similar in patients who received induction immunosuppressive treatment to those who did not (168.63±60.17ng/mL vs. 155.58±58.19ng/mL, p=0.294).
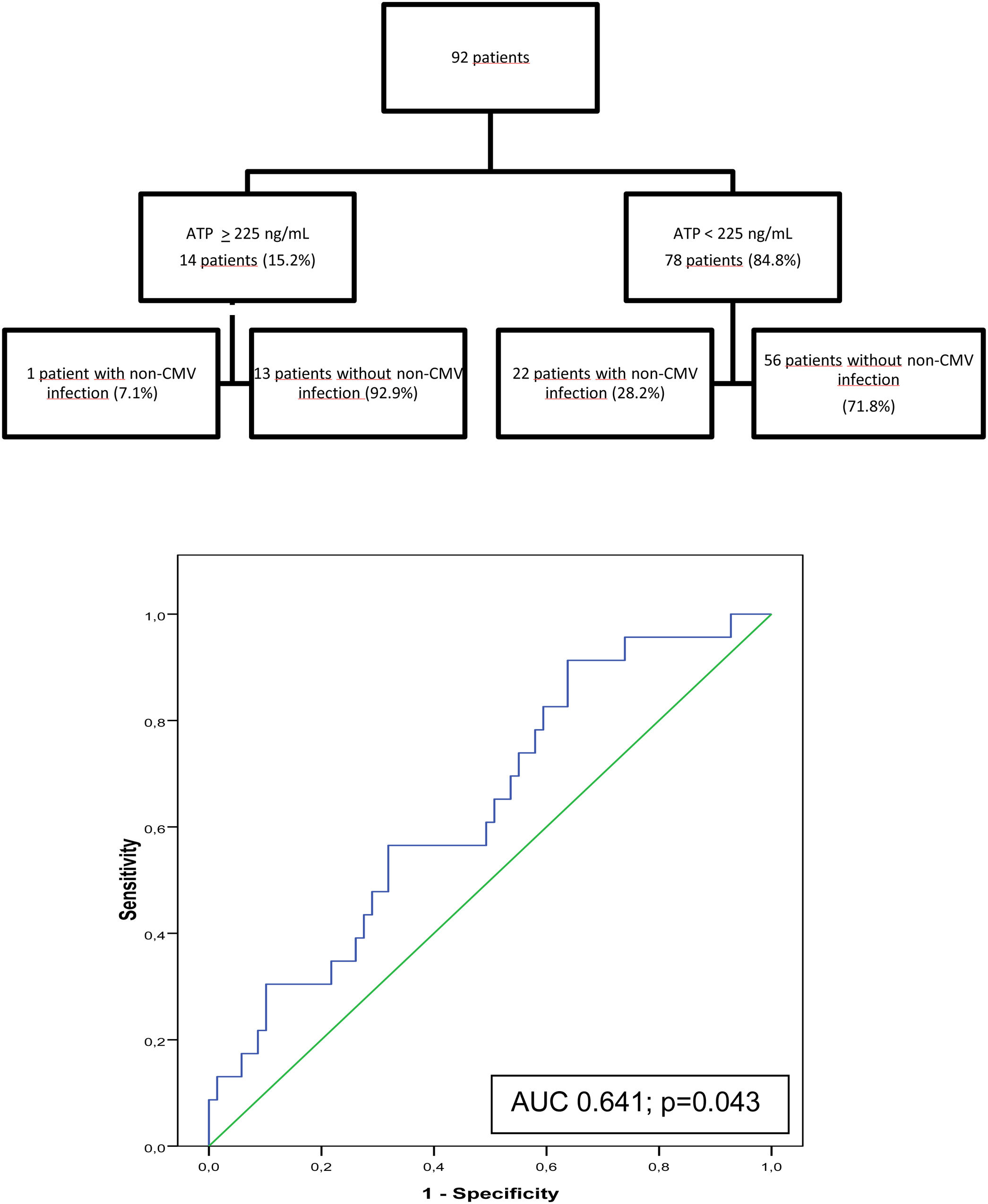
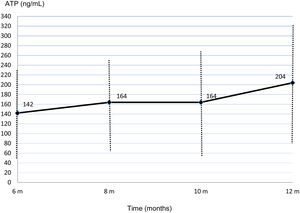
At 6 months post-transplant, a moderate immune response was detected in 14 of the 92 patients studied (15.2%) and a low response in 78 patients (84.8%). No patients showed a strong immune response. Only 1 of the 14 patients (7.1%) with moderate immune response developed an infection between 6 and 12 months after surgery compared with 22 of the 78 patients (28.2%) with low response. In the ROC curve analysis, the assay had a sensitivity of 95.7% and a specificity of 18.8% (AUC, 0.641; p=0.043). With an ATP cut-off of 225ng/mL, the PPV and NPV were 28.2%, and 92.9% respectively. Specificity improved at an ATP cut-off of<40ng/mL (86.36%) but sensitivity decreased (9.23%) (Fig. 2).
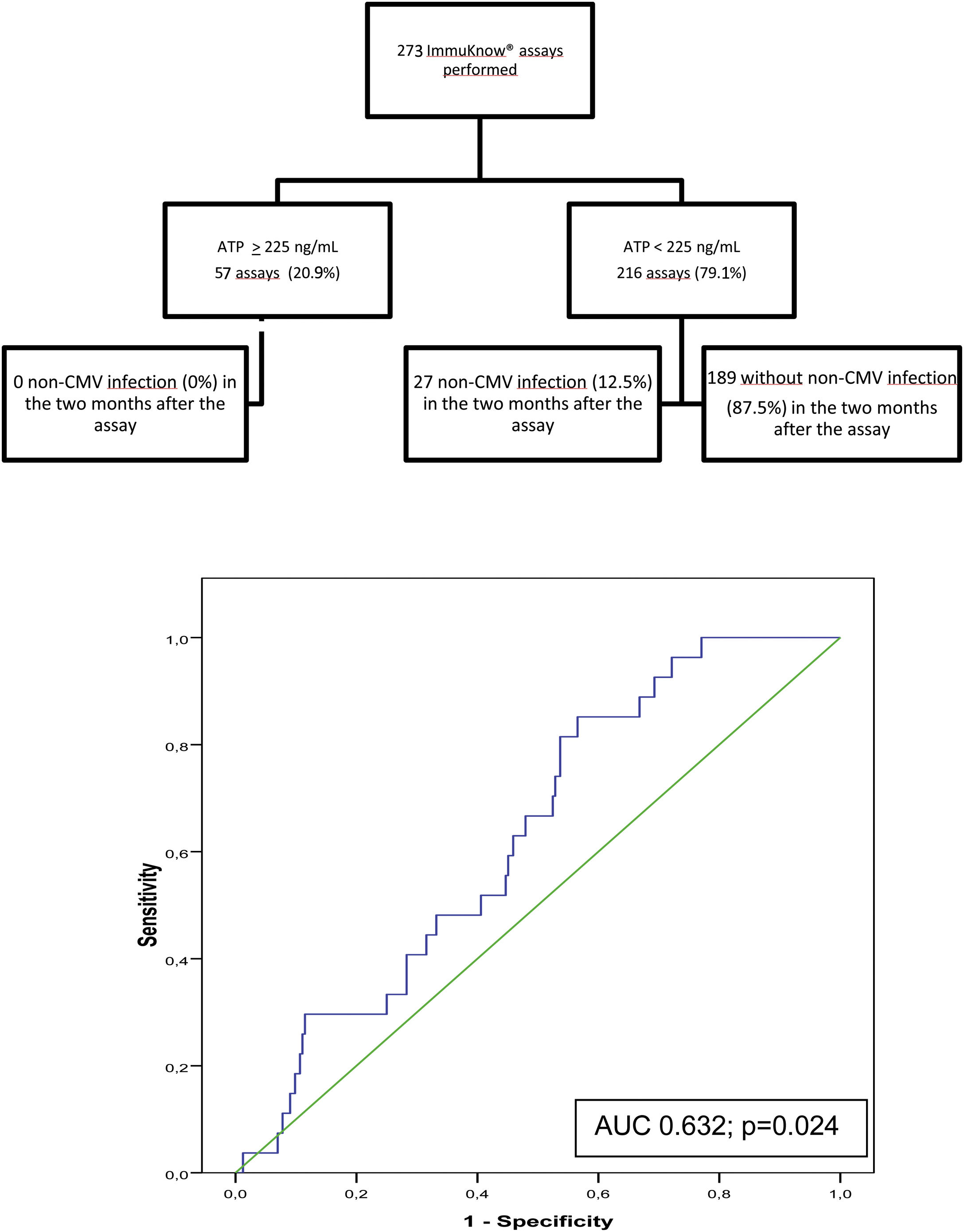
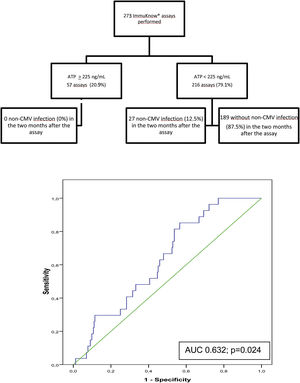
Of the 273 assays performed, a moderate immune response was detected in 55 samples (20.2%) and a low response in 216 (79.1%). Two samples showed a strong immune response (0.7%). The rate of infection during the 2 months following testing was 0% in patients with strong or moderate immune response and 12.5% in those with a low response. ROC curve analysis revealed a sensitivity of 100% and a specificity of 22.5%, (AUC, 0.632; p=0.024) with a PPV of 12.5%, and NPV of 100%% at an ATP cut-off of 225ng/mL. Specificity improved at an ATP cut-off of<40ng/mL (88.89%) and sensitivity decreased (9.02%) (Fig. 3).
Flow diagram of assayed samples from patients who developed non-CMV infection or not in the 2 months following ImmuKnow® testing, and ROC curve showing the sensitivity and specificity of the assay29
Between 6 and 12 months after surgery, 14 of the 92 patients studied (15.2%) had mean ATP values≥225ng/mL and 78 (84.8%) had mean ATP<225ng/mL. Acute rejection was recorded in 1 of the 14 patients with mean ATP≥225ng/mL (7.1%) and in 7 of the 77 patients with mean ATP<225ng/mL (9.1%, p=0.81). Similarly, there were no differences in respiratory function progress between the groups.
DiscussionLittle is known about the diagnostic value of the ImmuKnow® assay in lung transplantation.6–10 To our knowledge, this is the first multicenter prospective study to analyze the predictive value of ImmuKnow® for infections other than CMV in this type of transplant. We observed that patients with ImmuKnow® ATP values ≥225ng/mL had a very low risk of developing non-CMV infection both short term and medium term, with no increase in the acute rejection rate or worsening in the evolution of respiratory function. In the 2 months following performance of the assay, the NPV was 100%, and in the following 6 months it was 93%. However, the specificity and PPV were low. Therefore ImmuKnow® does not seem useful to predict episodes of non-CMV infection in lung transplant recipients, but could identify patients with a very low risk (ATP>225ng/mL) and help us define a target for an optimal immunosuppression.
The ImmuKnow® assay has been evaluated in lung transplant in a few studies with different designs to that of the present study. The novel aspects of our study are that it was designed to evaluate the sensitivity and specificity of the assay to predict which patients will go on to develop infections, that it included non-respiratory infections, and that it was a multicenter study. Bhorade et al.6 studied 143 samples from 57 lung transplant recipients. Like us, the authors observed that patients who developed infection had lower ImmuKnow® values, but unlike our study the assays were performed at the time of infection, not before. Husain et al.7 found that patients with bacterial pneumonia, viral infection, CMV disease, and fungal infection had lower ImmuKnow® values (also at time of infection) than uninfected patients and that ImmuKnow® values<100ng/mL were an independent risk factor for infection, with an odds ratio of 2.81. In a retrospective study, Shino et al.8 correlated ImmuKnow® values with findings from bronchoscopy. Their findings differed, in that ImmuKnow® values were similar in patients who developed infection and in those who did not. It should be noted that in this study, ImmuKnow® values were very high compared with those reported in other published studies.6,7,9,10 Even so, Shino et al.8 found that 40% of patients with ATP levels<225ng/mL developed an infection (odds ratio, 1.9).
This assay provides information on global T cell immunocompetence and risk of all types of opportunistic infection.7 However, Husain et al.7 observed than patients with CMV disease showed the lowest ImmuKnow® values, followed by those with fungal and then bacterial infections: this is probably because T cells are more involved in the control of CMV infection than in bacterial or fungal infections where innate immunity is the first line of defense. We therefore decided to study the predictive value of ImmuKnow® for infections other than CMV.
ImmuKnow® assay has been extensively studied in other types of SOT. Its value for predicting infection is controversial and varies with the organ transplanted. Discrepancies are probably due to differences in the incidence of infection between organs, immunosuppression protocols and study design.15 Overall, the sensitivity for predicting the risk of infection is high for liver transplant but lower for kidney transplant. In their meta-analysis, Ling et al.4 found that in 3 studies on liver transplant,16–18 sensitivity ranged from 81% to 100%, whereas in 3 studies on kidney transplant,19–21 sensitivity was between 21% and 68%. Specificity for both organs was similar, between 60% and 79% in liver transplant and between 74% and 100% in kidney transplant. In another meta-analysis, Rodrigo et al.5,16,18,22,23 found sensitivity to be 83% and specificity 75% for liver transplant. Huskey et al.21 also in a retrospective study with 583 renal transplant recipients at a single center, observed a lack of sensitivity and specificity as a predictive test for infection. Kowalski et al.15 observed in a meta-analysis with 504 SOT recipients that ATP levels around 280ng/mL may protect against allograft rejection and infection.
In our study, tacrolimus levels were no higher in patients who developed infection than in those who did not. Similarly, they did not correlate with ImmuKnow® levels. This finding is well documented6,24,25 and points to inappropriate monitoring of levels of immunosuppression based only on pharmacokinetic parameters. In addition to ImmuKnow®, other assays have been proposed for monitoring the immune response (eg, cytokine genetic polymorphisms, mixed lymphocyte reaction, enzyme-linked immunosorbent spot assay, Quantiferon assay or monitoring of nuclear factor of activated T cells-regulated gene expression), although they have not been implemented in clinical practice, since they involve complicated laboratory procedures or are subject to problems of reproducibility and cost. However, the main impediment to their use in clinical practice is the lack of prospective studies and randomized clinical trials.2,3,26–28 Ravaioli et al.25 performed a randomized controlled trial in liver transplant recipients in which they modified the dose of tacrolimus according to the ImmuKnow® result, with a 25% dose reduction if ATP levels were<130ng/mL and a 25% dose increase if they were>450ng/mL. With this strategy, patients developed fewer infections than in the control group (42% vs 54.9%) and had better 1-year survival (95% vs 82%). It would be interesting to perform a study with a similar design in lung transplant recipients, where infection plays an even more important role. In fact, reducing the number of infections is essential if we are to improve the outcomes of lung transplantation. Randomized clinical trials in which immunosuppressive therapy is adjusted according to ImmuKnow® or other immunological monitoring assays are needed.
Studies on acute rejection performed in liver transplantation4,16–18 have shown high specificity (94–100%) and low sensitivity (9–50%) for ImmuKnow® with a cut-off point of ATP>525ng/mL. The results are poorer in kidney transplantation, with a specificity of 65–80% and a sensitivity of 33–23%. In lung transplantation, Shino et al.8 reported a sensitivity of 45% and a specificity of 79% with this cut-off point. In our study, we cannot draw conclusions because only two samples out of 271 were above this limit; we did however observe the same acute rejection rate in patients with mean ImmuKnow® values of ATP≥225ng/mL and <225ng/mL during the study period.
With respect to pulmonary function, there were no differences between patients with ATP levels≥225ng/mL and patients with lower levels. However, follow-up was too short to draw conclusions on changes in pulmonary function.
The main limitations of the present study are those of multicenter observational studies. Although we attempted to collect all variables prospectively in order to minimize potential bias, some variables are difficult to record and analyze. For example, one physician may address a particular clinical situation differently from another, or there may be differences between centers in terms of prophylaxis, immunosuppression, and patient follow-up. Our study is also limited by the short follow-up period, although this was during a period when the patient is still very susceptible to infections, i.e., between 6 and 12 months after surgery. We excluded the first 6 months because some postoperative variables can also play a role in the development of infection (for example problems in bronchial anastomosis, leukopenia due to valganciclovir prophylaxis, and different fungal or bacterial prophylaxis between centers). Therefore, the predictive value of ImmuKnow® in these first 6 months is unclear. Finally, our study was limited by potential variability in the immunoassays used; we attempted to minimize this variability by using a central laboratory.
In conclusion, ImmuKnow® does not seem useful to predict episodes of non-CMV infection in lung transplant recipients, but could identify patients with a very low risk. ImmuKnow® could help us to adjust the immunosuppressive treatment, reducing the dose of immunosuppressants when the ImmuKnow® values are very low. However, this should be evaluated with clinical trials designed for this purpose.
Author contributionsVM, SGO, RC, VLP, JMC, JR, AS contributed to the design; VM, SGO, HS, RC, PU coordinated the study; VM, CB, MLM, PJM, JPO, VLP, RA, JMC, JMV, JR, RL, PU, JE, AS contributed to the data collection; SR, RC, ILA contributed to sample analysis; VM, SGO, HS, ILA, AM contributed to data analysis; VM wrote the manuscript; VM, VLP, JMC, DI, JR, PU, AS contributed to the revision of the manuscript.
Funding sourceThis work was supported by Roche Farma S.A.
Conflict of interestThe authors have no conflict of interests related to this manuscript to disclose.
The authors thank Sonia López and Rosa Llòria and for their assistance and technical help and Nuria Pajuelo for her work in the statistical analysis.