Pneumonia is considered an independent entity in chronic obstructive pulmonary disease (COPD), to be distinguished from an infectious exacerbation of COPD. The aim of this study was to analyze the clinical characteristics and progress of the exacerbation of COPD (ECOPD) compared to pneumonia in COPD (PCOPD) patients requiring hospitalization.
Patients and methodsProspective, longitudinal, observational cohort study including 124 COPD patients requiring hospital admission for lower respiratory tract infection. Patients were categorized according to presence of ECOPD (n=104) or PCOPD (n=20), depending on presence of consolidation on X-ray. Demographic, clinical, laboratory, microbiological and progress variables were collected.
ResultsPatients with ECOPD showed more severe respiratory disease according to the degree of obstruction (P<.01) and need for oxygen therapy (P<.05). PCOPD patients showed increased presence of fever (P<.05), higher blood pressure (P<.001), more laboratory abnormalities (P<.05; leukocytosis, elevated CRP, low serum albumin) and increased presence of crepitus (P<.01). Microbiological diagnosis was achieved in 30.8% of cases of ECOPD and 35% of PCOPD; sputum culture yielded the highest percentage of positive results, predominantly Pseudomonas aeruginosa. Regarding the progress of the episode, no differences were found in hospital stay, need for ICU or mechanical ventilation.
ConclusionsOur data confirm clinical and analytical differences between ECOPD and PCOPD in patients who require hospital admission, while there were no differences in subsequent progress.
La neumonía se considera una entidad propia, diferente a la exacerbación de la enfermedad pulmonar obstructiva crónica (EPOC) de causa infecciosa. El objetivo de nuestro estudio fue analizar las características clínicas y la evolución según se presentara una agudización de la EPOC (AEPOC) o una neumonía (NEPOC) en los pacientes con EPOC que precisaban un ingreso hospitalario.
Pacientes y métodosEstudio de cohortes, prospectivo, longitudinal y observacional que incluyó 124 pacientes con EPOC que precisaron ingreso hospitalario por una infección respiratoria baja. Se categorizaron según presentaran una AEPOC (n=104) o una NEPOC (n=20), en función de la aparición de condensación radiológica. Recogida de variables demográficas, clínicas, de laboratorio, microbiológicas y evolutivas.
ResultadosLos pacientes con AEPOC mostraban mayor gravedad de la enfermedad respiratoria según el grado de obstrucción (p<0,01) y necesidad de oxigenoterapia crónica (p<0,05). Los pacientes con NEPOC mostraban mayor presencia de fiebre (p<0,05), mayor hipotensión arterial (p<0,001), mayor alteración analítica (p<0,05; leucocitosis, elevación de la PCR, hipoalbuminemia), así como mayor presencia de crepitantes (p<0,01). El diagnóstico microbiológico se obtuvo en el 30,8% de los casos de AEPOC y en el 35% de las NEPOC, siendo el cultivo de esputo la técnica con mayor porcentaje de resultados positivos, mostrando una preponderancia de Pseudomonas aeruginosa. La evolución del episodio no mostró diferencias en la estancia hospitalaria, ni la necesidad de UCI o ventilación mecánica.
ConclusionesNuestros datos confirman diferencias clínicas y analíticas entre una AEPOC y una NEPOC en los pacientes que precisan ingreso hospitalario, aunque sin diferencias en la evolución posterior.
Exacerbations are common in the natural history of patients with chronic obstructive pulmonary disease (COPD). Moreover, these patients have been shown to have a higher rate of hospital admission due to respiratory tract infection, which increases with the severity of the disease.1 Lower respiratory tract infection (LRTI) is one of the most common causes of decompensation in COPD in hospitalized patients, accounting for 51%–70% of exacerbations.2 These episodes are difficult to define, and there are no universally accepted clinical criteria, although the combination of symptoms described by Anthonisen3 (increased dyspnea, sputum production and sputum purulence) has been used to identify cases with an infectious etiology.
Exacerbation of chronic obstructive pulmonary disease (ECOPD) and community-acquired pneumonia are common respiratory diseases that contribute to patient hospitalization and mortality. There is considerable debate on whether the coexistence of these two entities might be a factor in increasing mortality. The results of studies published to date reporting on mortality have been inconsistent, probably due to their considerable heterogeneity.4
COPD is also one of the most common comorbidities in pneumonia in most studies, occurring in 30% of patients who require hospitalization,5 and in up to 50% of cases with severe pneumonia who require admission to an Intensive Care Unit (ICU).6 Likewise, the incidence of pneumonia in patients with COPD is almost twice that of the general population, correlating positively with the level of obstruction.7
Community-acquired pneumonia has hitherto been included as one of the causes of acute exacerbation (according to some guidelines8). It is currently considered an infectious comorbidity, differentiating it from ECOPD.9 The aim of our study was to analyze the clinical characteristics, laboratory data, etiology and progress of patients requiring hospitalization, comparing patients with exacerbation of chronic obstructive pulmonary disease (ECOPD) versus patients with pneumonia in COPD (PCOPD).
Patients and MethodsPatient SelectionA prospective, longitudinal, observational cohort study was conducted in patients seen in routine clinical practice in a general hospital (Hospital de Mataró) with a catchment area of 240,000 inhabitants.
Adult patients of any age diagnosed with COPD who required hospitalization for an LRTI between 1st October and 31st December 2009 were identified from the censuses of the Internal Medicine department, Respiratory Medicine department and Short Stay Unit. Patients with known COPD according to spirometric data prior to admission were included. Spirometry was performed on the remaining patients on discharge when there was suspected COPD, clinical criteria of chronic bronchitis, or cumulative cigarette smoking in excess of 10 packets-year. The diagnosis was confirmed in accordance with recognized COPD guidelines (post-bronchodilator FEV1/FVC<0.7).9,10
At least 2 of Anthonisen's criteria3 were used to include the case as ECOPD of probable infectious origin, and the presence of a new radiological condensation was required for the diagnosis of PCOPD, with follow-up X-ray after 1 month. Radiography results were interpreted by our Radiology department according to routine clinical practice, and confirmed by one of the study investigators (Internal Medicine specialist).
Patients were treated by the usual medical team in each department, and the data were collected by the investigators; diagnosis of pneumonia was confirmed by radiological findings. Clinical management of patients followed current clinical practice guidelines.
As this was a non-interventional observational study, neither assessment by an ethics committee nor patient informed consent was requested.
ProceduresDemographic variables such as age, sex, smoking habits, influenza and pneumococcal vaccination, and history of exacerbation and pneumonia in the previous year were collected. Long-term home oxygen therapy (LTOT) and long-term use of corticoids and antibiotics (according to their occasional or daily use in a stable phase in the 3 months prior to the current episode) were recorded. Any accompanying comorbidity was assessed, noting the most common comorbid conditions and the Charlson index. Functional capacity was assessed using the Barthel index.
The severity of the lung disease was assessed on the basis of spirometric values obtained when the patient's condition was stable, according to GOLD10 criteria and the baseline dyspnea scale (mMRC scale11).
A series of clinical–biological parameters were obtained from all patients on admission (temperature, heart rate, respiratory rate, basal pulse oximetry, blood pressure, basal arterial blood gas, hemoglobin, white cell count, C-reactive protein (CRP), urea, total protein, albumin, protein electrophoresis, glucose, creatine kinase and fibrinogen).
The period between symptom onset and arrival at the Emergency department was also determined, and dysthermia, chills, fever and purulent sputum were recorded.
Routine microbiology tests for etiological diagnosis were carried out at the attending physician's discretion, including conventional Gram staining and sputum culture, serial blood cultures and urinary antigens for Streptococcus pneumoniae and Legionella pneumophilia. As the study period fell within the flu pandemic, polymerase chain reaction (PCR) techniques were used to detect the influenza virus following the clinical practice protocol established as a result of the situation.12 This restricted clinical sample collection to serious cases of infection described by the Spanish Ministry of Health as follows: severe clinical symptoms consistent with the pandemic virus (H1N1) requiring hospital admission and suspected pneumonia due to the pandemic virus. In clinical practice, infection due to influenza virus A was suspected when the patient had temperature greater than 38°C with acute respiratory infection, or when pneumonia of unknown etiology was diagnosed, or in case of death due to respiratory disease.
Finally, data related to hospitalization, such as length of hospital stay, resources used (admission to ICU and need for invasive mechanical ventilation), therapeutic failure and need to change the antibiotic due to proven resistance were included. Mortality during admission was also determined.
Data AnalysisUsing the data obtained, a descriptive analysis was performed of all the study variables using SPSS software 14.0. Qualitative variables were expressed as absolute frequencies and percentages (%), while the quantitative variables were expressed as means±standard deviations (SD), or medians [25–75 percentiles]. Comparison of means was performed using the Student's t-test for independent samples, applying the Bonferroni correction when indicated. The Kolmogorov test was used to evaluate the normality of the data, and the Mann–Whitney U-test was applied for variables that did not meet normality criteria. The Chi-square test or Fisher's exact test was used to compare proportions. In all cases, a two-sided hypothesis was considered, with P<.05 indicating statistically significant results.
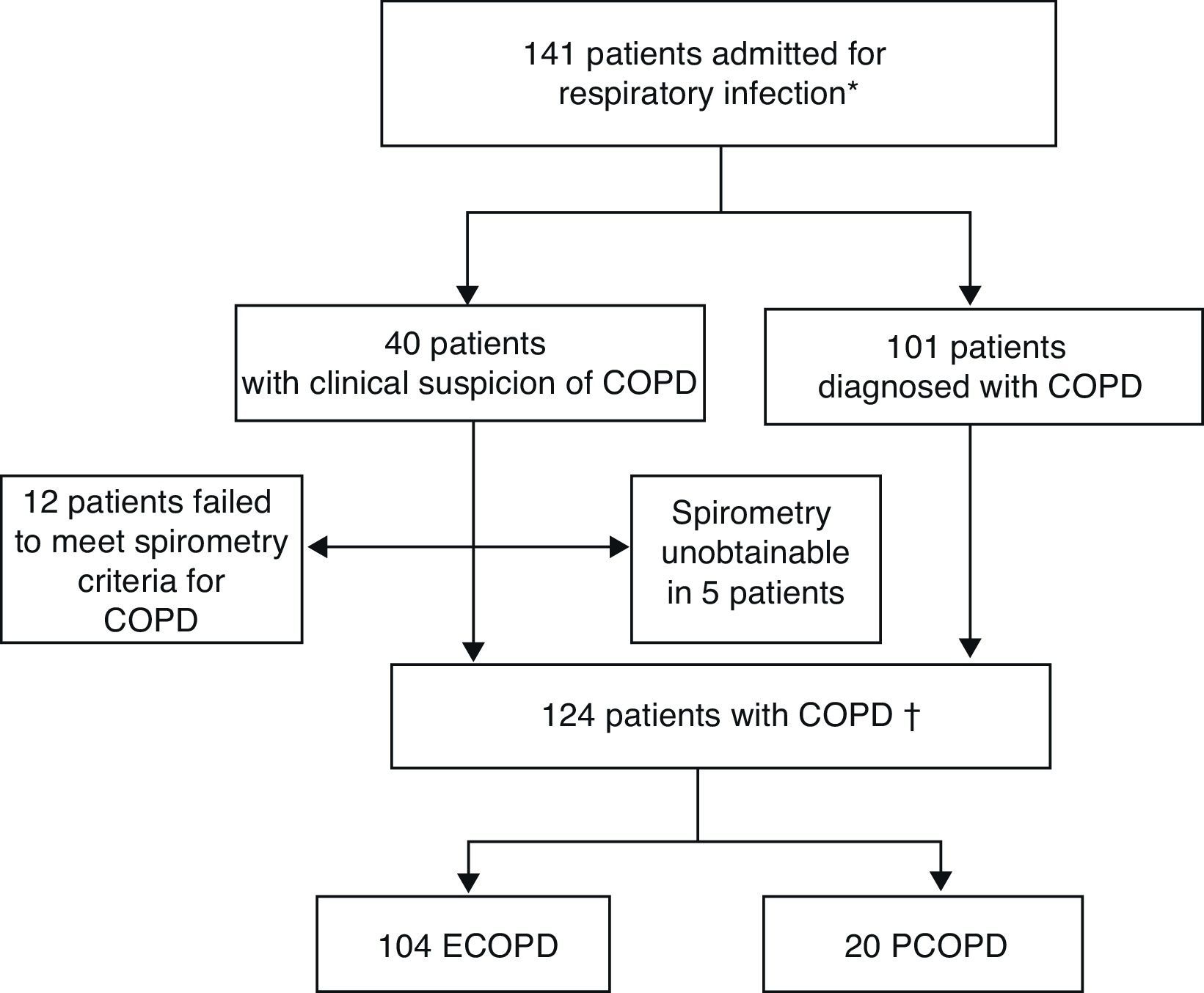
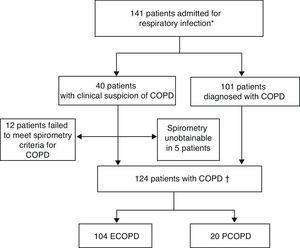
ResultsDuring the study period, 141 patients with a diagnosis or clinical suspicion of COPD (acute exacerbation or pneumonia) were admitted to hospital for acute respiratory infection. The diagnosis of COPD could not be confirmed in 12 patients, as they did not meet spirometric criteria. One hundred and 24 episodes were eventually included: 104 for ECOPD and 20 for PCOPD (Fig. 1).
Inclusion of patients in study.
ECOPD: exacerbation of chronic obstructive pulmonary disease; PCOPD: pneumonia with chronic obstructive pulmonary disease.
*Patients with spirometric diagnosis of COPD or clinical criteria without diagnostic spirometry at the time of admission.
†Patients with spirometric diagnosis of COPD.
Patients were mostly admitted to the Internal Medicine department (64.5%), followed by the Respiratory Medicine department (31.5%), and finally the Short Stay Unit (4%).
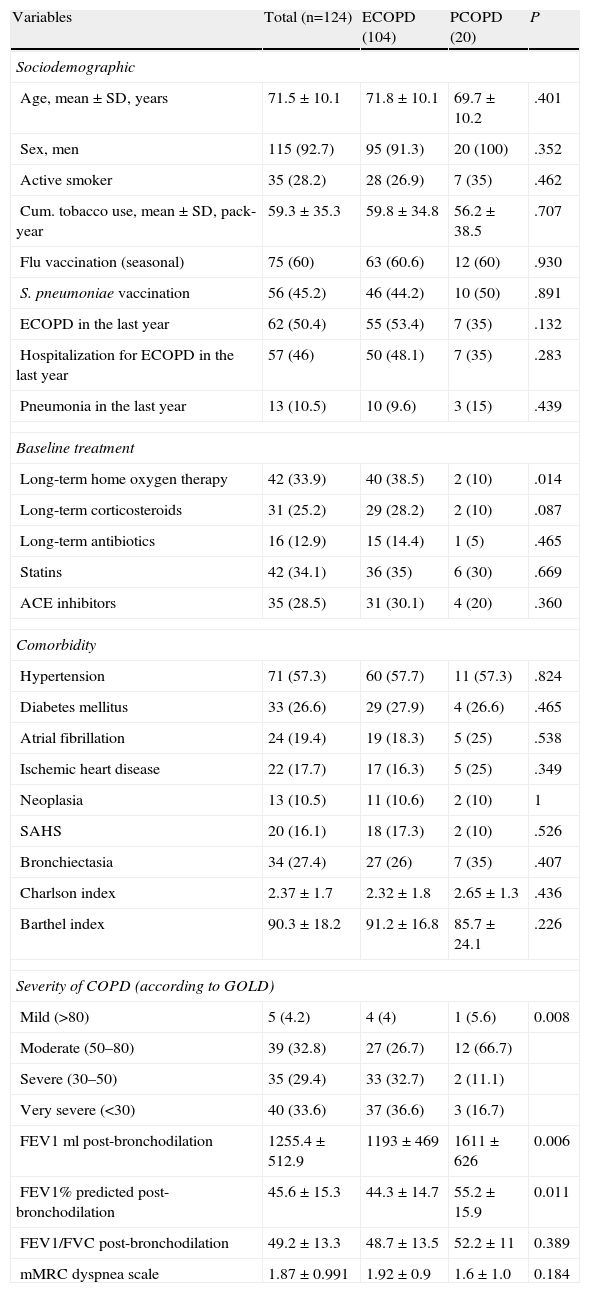
Patients’ general clinical characteristics are described in Table 1, according to groups. There were no differences between groups in terms of demographic data, comorbidity and functional capacity, or patient vaccination.
Patient Clinical Characteristic.
| Variables | Total (n=124) | ECOPD (104) | PCOPD (20) | P |
| Sociodemographic | ||||
| Age, mean±SD, years | 71.5±10.1 | 71.8±10.1 | 69.7±10.2 | .401 |
| Sex, men | 115 (92.7) | 95 (91.3) | 20 (100) | .352 |
| Active smoker | 35 (28.2) | 28 (26.9) | 7 (35) | .462 |
| Cum. tobacco use, mean±SD, pack-year | 59.3±35.3 | 59.8±34.8 | 56.2±38.5 | .707 |
| Flu vaccination (seasonal) | 75 (60) | 63 (60.6) | 12 (60) | .930 |
| S. pneumoniae vaccination | 56 (45.2) | 46 (44.2) | 10 (50) | .891 |
| ECOPD in the last year | 62 (50.4) | 55 (53.4) | 7 (35) | .132 |
| Hospitalization for ECOPD in the last year | 57 (46) | 50 (48.1) | 7 (35) | .283 |
| Pneumonia in the last year | 13 (10.5) | 10 (9.6) | 3 (15) | .439 |
| Baseline treatment | ||||
| Long-term home oxygen therapy | 42 (33.9) | 40 (38.5) | 2 (10) | .014 |
| Long-term corticosteroids | 31 (25.2) | 29 (28.2) | 2 (10) | .087 |
| Long-term antibiotics | 16 (12.9) | 15 (14.4) | 1 (5) | .465 |
| Statins | 42 (34.1) | 36 (35) | 6 (30) | .669 |
| ACE inhibitors | 35 (28.5) | 31 (30.1) | 4 (20) | .360 |
| Comorbidity | ||||
| Hypertension | 71 (57.3) | 60 (57.7) | 11 (57.3) | .824 |
| Diabetes mellitus | 33 (26.6) | 29 (27.9) | 4 (26.6) | .465 |
| Atrial fibrillation | 24 (19.4) | 19 (18.3) | 5 (25) | .538 |
| Ischemic heart disease | 22 (17.7) | 17 (16.3) | 5 (25) | .349 |
| Neoplasia | 13 (10.5) | 11 (10.6) | 2 (10) | 1 |
| SAHS | 20 (16.1) | 18 (17.3) | 2 (10) | .526 |
| Bronchiectasia | 34 (27.4) | 27 (26) | 7 (35) | .407 |
| Charlson index | 2.37±1.7 | 2.32±1.8 | 2.65±1.3 | .436 |
| Barthel index | 90.3±18.2 | 91.2±16.8 | 85.7±24.1 | .226 |
| Severity of COPD (according to GOLD) | ||||
| Mild (>80) | 5 (4.2) | 4 (4) | 1 (5.6) | 0.008 |
| Moderate (50–80) | 39 (32.8) | 27 (26.7) | 12 (66.7) | |
| Severe (30–50) | 35 (29.4) | 33 (32.7) | 2 (11.1) | |
| Very severe (<30) | 40 (33.6) | 37 (36.6) | 3 (16.7) | |
| FEV1ml post-bronchodilation | 1255.4±512.9 | 1193±469 | 1611±626 | 0.006 |
| FEV1% predicted post-bronchodilation | 45.6±15.3 | 44.3±14.7 | 55.2±15.9 | 0.011 |
| FEV1/FVC post-bronchodilation | 49.2±13.3 | 48.7±13.5 | 52.2±11 | 0.389 |
| mMRC dyspnea scale | 1.87±0.991 | 1.92±0.9 | 1.6±1.0 | 0.184 |
ECOPD, exacerbation of COPD; PCOPD, pneumonia in a patient with COPD; Pack-year, packets-year; ACE, angiotensin-converting enzyme; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SAHS, sleep apnea-hypopnea syndrome; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
Results presented as absolute numbers (percentage), or mean±standard deviation when applicable.
There was a greater history of exacerbation in the ECOPD group and pneumonia in the PCOPD group in the previous year, but this did not reach statistical significance.
The basic treatment was similar, except in patients in the ECOPD group, who were treated with LTOT more often than patients in the PCOPD group (40% vs 10%; P=.014).
Recent spirometry results (<6 months) were recorded in 119 patients. According to these data, patients in the ECOPD group were found to have a greater degree of obstruction as assessed by post-bronchodilator FEV1 (% predicted, 44.3±14.7 vs 55.2±15.9, P=.011); a significantly higher percentage of patients were classified as severe-very severe (P=.008) using GOLD10 classification of the obstruction. There were no differences between groups according to the mMRC dyspnea scale.
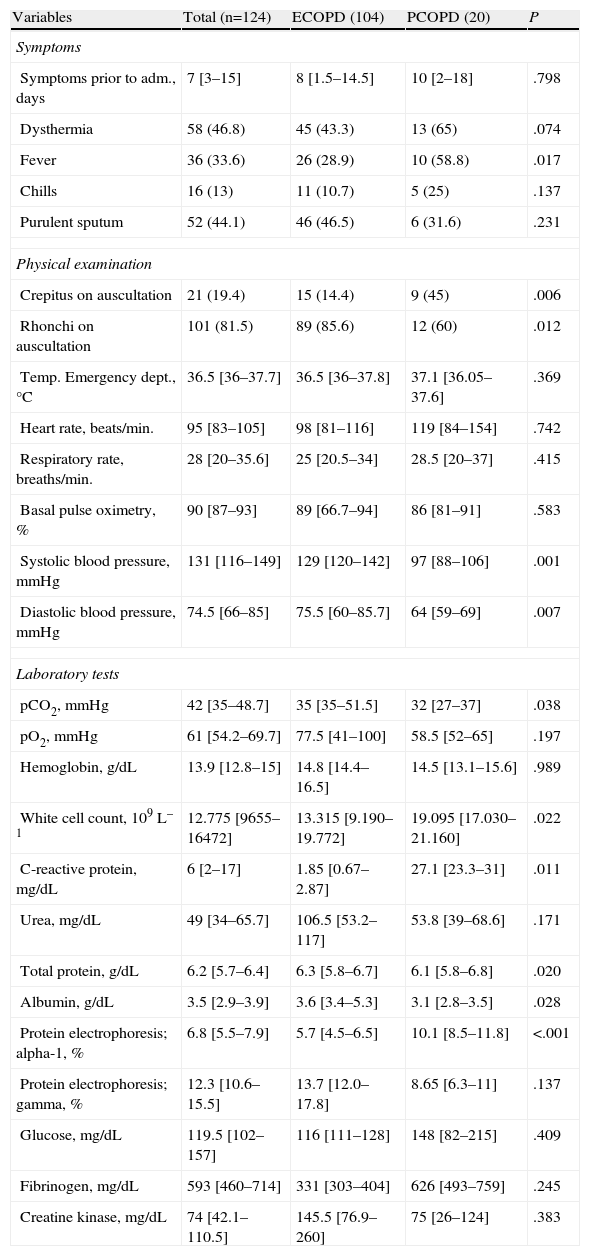
Table 2 shows the clinical characteristics of the episode. Patients in the PCOPD group had a higher incidence of fever (P=.017), and also reported dysthermia, chills and purulent sputum more often, although this did not reach statistical significance. In the physical examination, there was a higher incidence of crepitus in the PCOPD group (45% vs 14.4%, P=.006) and rhonchi in the ECOPD group (85.6% vs 60%; P=.012). Hemodynamic data showed greater alteration in blood pressure on arrival at the Emergency department in the PCOPD group (lower systolic and diastolic blood pressure, P=.007), with the remaining vital signs being similar in both groups, including temperature taken on examination.
Characteristics of the Episode due to Respiratory Infection.
| Variables | Total (n=124) | ECOPD (104) | PCOPD (20) | P |
| Symptoms | ||||
| Symptoms prior to adm., days | 7 [3–15] | 8 [1.5–14.5] | 10 [2–18] | .798 |
| Dysthermia | 58 (46.8) | 45 (43.3) | 13 (65) | .074 |
| Fever | 36 (33.6) | 26 (28.9) | 10 (58.8) | .017 |
| Chills | 16 (13) | 11 (10.7) | 5 (25) | .137 |
| Purulent sputum | 52 (44.1) | 46 (46.5) | 6 (31.6) | .231 |
| Physical examination | ||||
| Crepitus on auscultation | 21 (19.4) | 15 (14.4) | 9 (45) | .006 |
| Rhonchi on auscultation | 101 (81.5) | 89 (85.6) | 12 (60) | .012 |
| Temp. Emergency dept., °C | 36.5 [36–37.7] | 36.5 [36–37.8] | 37.1 [36.05–37.6] | .369 |
| Heart rate, beats/min. | 95 [83–105] | 98 [81–116] | 119 [84–154] | .742 |
| Respiratory rate, breaths/min. | 28 [20–35.6] | 25 [20.5–34] | 28.5 [20–37] | .415 |
| Basal pulse oximetry, % | 90 [87–93] | 89 [66.7–94] | 86 [81–91] | .583 |
| Systolic blood pressure, mmHg | 131 [116–149] | 129 [120–142] | 97 [88–106] | .001 |
| Diastolic blood pressure, mmHg | 74.5 [66–85] | 75.5 [60–85.7] | 64 [59–69] | .007 |
| Laboratory tests | ||||
| pCO2, mmHg | 42 [35–48.7] | 35 [35–51.5] | 32 [27–37] | .038 |
| pO2, mmHg | 61 [54.2–69.7] | 77.5 [41–100] | 58.5 [52–65] | .197 |
| Hemoglobin, g/dL | 13.9 [12.8–15] | 14.8 [14.4–16.5] | 14.5 [13.1–15.6] | .989 |
| White cell count, 109L–1 | 12.775 [9655–16472] | 13.315 [9.190–19.772] | 19.095 [17.030–21.160] | .022 |
| C-reactive protein, mg/dL | 6 [2–17] | 1.85 [0.67–2.87] | 27.1 [23.3–31] | .011 |
| Urea, mg/dL | 49 [34–65.7] | 106.5 [53.2–117] | 53.8 [39–68.6] | .171 |
| Total protein, g/dL | 6.2 [5.7–6.4] | 6.3 [5.8–6.7] | 6.1 [5.8–6.8] | .020 |
| Albumin, g/dL | 3.5 [2.9–3.9] | 3.6 [3.4–5.3] | 3.1 [2.8–3.5] | .028 |
| Protein electrophoresis; alpha-1, % | 6.8 [5.5–7.9] | 5.7 [4.5–6.5] | 10.1 [8.5–11.8] | <.001 |
| Protein electrophoresis; gamma, % | 12.3 [10.6–15.5] | 13.7 [12.0–17.8] | 8.65 [6.3–11] | .137 |
| Glucose, mg/dL | 119.5 [102–157] | 116 [111–128] | 148 [82–215] | .409 |
| Fibrinogen, mg/dL | 593 [460–714] | 331 [303–404] | 626 [493–759] | .245 |
| Creatine kinase, mg/dL | 74 [42.1–110.5] | 145.5 [76.9–260] | 75 [26–124] | .383 |
ECOPD, exacerbation of COPD; PCOPD, pneumonia in a patient with COPD; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen.
Results presented as absolute numbers (percentage), or median [5–75 percentiles] when applicable.
According to laboratory data at the time of admission, patients in the ECOPD group had higher partial pressure of carbon dioxide (pCO2) in the arterial blood gas (P=.038). In contrast, patients in the PCOPD group had a higher white cell count (19095×109L–1 vs 13315×109L–1; P=.007), high CRP (27.1mg/dl vs 1.85mg/dl; P=.011), hypoalbuminemia (3.1mg/dl vs 3.6mg/dl; P=.028) and hypoproteinemia (6.1mg/dl vs 6.3mg/dl; P=.02), as well as an increase in the alpha-1-globulin fraction on serum protein electrophoresis (10.1% vs 5.7%; P=.001).
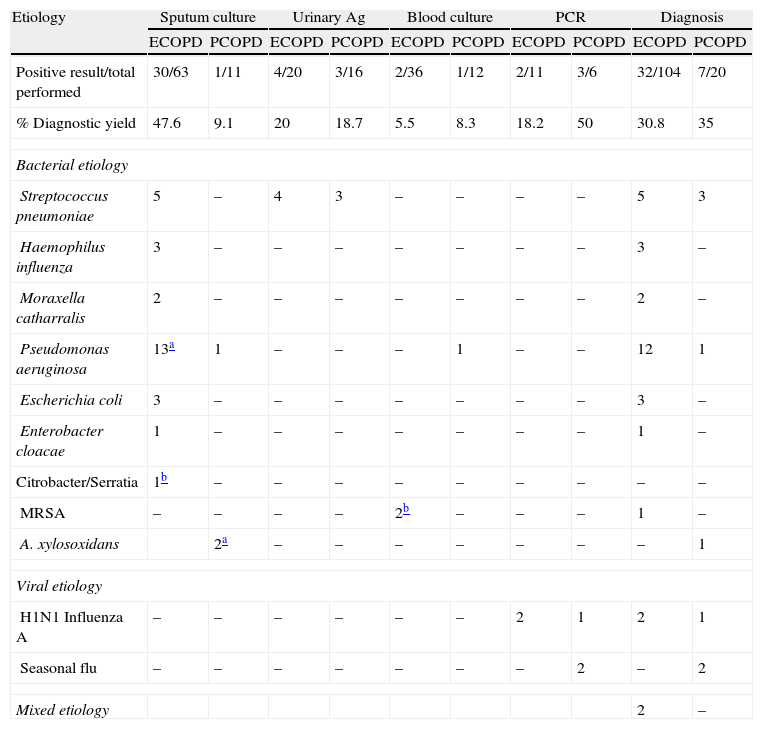
Table 3 shows the diagnostic tests performed and the percentage of positive results for each group. The percentage of positive sputum cultures was higher in the ECOPD group (47.6% vs 9.1%; P=.05), while the percentage of positive throat swabs for influenza was higher in cases of PCOPD (50% vs 18.2%; P=.023).
Microbiology. Diagnostic Methods and Etiology in Exacerbation of COPD (ECOPD) and Pneumonia in COPD (PCOPD).
| Etiology | Sputum culture | Urinary Ag | Blood culture | PCR | Diagnosis | |||||
| ECOPD | PCOPD | ECOPD | PCOPD | ECOPD | PCOPD | ECOPD | PCOPD | ECOPD | PCOPD | |
| Positive result/total performed | 30/63 | 1/11 | 4/20 | 3/16 | 2/36 | 1/12 | 2/11 | 3/6 | 32/104 | 7/20 |
| % Diagnostic yield | 47.6 | 9.1 | 20 | 18.7 | 5.5 | 8.3 | 18.2 | 50 | 30.8 | 35 |
| Bacterial etiology | ||||||||||
| Streptococcus pneumoniae | 5 | – | 4 | 3 | – | – | – | – | 5 | 3 |
| Haemophilus influenza | 3 | – | – | – | – | – | – | – | 3 | – |
| Moraxella catharralis | 2 | – | – | – | – | – | – | – | 2 | – |
| Pseudomonas aeruginosa | 13a | 1 | – | – | – | 1 | – | – | 12 | 1 |
| Escherichia coli | 3 | – | – | – | – | – | – | – | 3 | – |
| Enterobacter cloacae | 1 | – | – | – | – | – | – | – | 1 | – |
| Citrobacter/Serratia | 1b | – | – | – | – | – | – | – | – | – |
| MRSA | – | – | – | – | 2b | – | – | – | 1 | – |
| A. xylosoxidans | 2a | – | – | – | – | – | – | – | 1 | |
| Viral etiology | ||||||||||
| H1N1 Influenza A | – | – | – | – | – | – | 2 | 1 | 2 | 1 |
| Seasonal flu | – | – | – | – | – | – | – | 2 | – | 2 |
| Mixed etiology | 2 | – | ||||||||
ECOPD, exacerbation of COPD; PCOPD, pneumonia in a patient with COPD; Urinary Ag, immunochromatographic urine assay for S. pneumoniae (immunochromatography for Legionella pneumophilia was performed at the same time, but all test results were negative: 20 in the ECOPD group and 16 in the PCOPD group); PCR, determination of nucleic acids using polymerase chain reaction techniques; MRSA: methicillin-resistant Staphylococcus aureus.
The microbiological diagnosis showed no differences between the groups. There were 32 positive results (30.8%) in the ECOPD group: Pseudomonas aeruginosa in 12 cases (37.5%), S. pneumoniae in 5 cases (15.6%), H. influenzae in 3 cases (9.4%), Escherichia coli in 3 cases (9.4%), Moraxella catharralis in 2 cases (6.25%), influenza virus A in 2 cases (6.25%), and 2 other cases of mixed etiology. There were 7 positive results in the PCOPD group: 3 cases (42.8%) of S. pneumoniae, 3 (42.8%) of influenza, and 1 case of P. aeruginosa (14.3%).
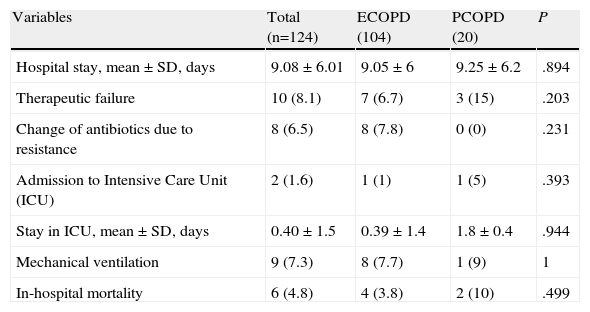
Finally, patient progress did not differ between groups. There were no differences in length of hospital stay, admission to the Intensive Care Unit, need for mechanical ventilation or in-hospital mortality. The data are shown in Table 4.
Evolution of the Episode due to Respiratory Infection.
| Variables | Total (n=124) | ECOPD (104) | PCOPD (20) | P |
| Hospital stay, mean±SD, days | 9.08±6.01 | 9.05±6 | 9.25±6.2 | .894 |
| Therapeutic failure | 10 (8.1) | 7 (6.7) | 3 (15) | .203 |
| Change of antibiotics due to resistance | 8 (6.5) | 8 (7.8) | 0 (0) | .231 |
| Admission to Intensive Care Unit (ICU) | 2 (1.6) | 1 (1) | 1 (5) | .393 |
| Stay in ICU, mean±SD, days | 0.40±1.5 | 0.39±1.4 | 1.8±0.4 | .944 |
| Mechanical ventilation | 9 (7.3) | 8 (7.7) | 1 (9) | 1 |
| In-hospital mortality | 6 (4.8) | 4 (3.8) | 2 (10) | .499 |
ECOPD: exacerbation of COPD: PCOPD: pneumonia in a patient with COPD.
Results presented as absolute numbers (percentage), or mean±standard deviation when applicable.
Therapeutic failure: therapeutic failure was considered as the onset of hemodynamic changes (1 case in ECOPD), worsening of respiratory failure (5 cases in ECOPD and 2 cases in PCOPD), onset of serious side effects or lack of response to treatment (1 case in ECOPD and another case in PCOPD).
Our study confirms that the clinical characteristics of PCOPD differ from ECOPD on admission, with greater systemic inflammation in the respiratory infection episode (fever, hemodynamic changes and greater elevation of biological markers), although the underlying respiratory disease was less severe (lower level of obstruction and less severe chronic respiratory failure).
Therefore, more severe, more hypoxemic COPD patients with bacterial colonization are more likely to present with COPD exacerbation and be admitted more often.
We also found microbiological differences, with S. pneumoniae predominating in PCOPD, and P. aeruginosa in ECOPD, as well as differences in terms of the percentage of positive diagnostic test results. Finally, despite the differences, we were unable to identify a more serious prognosis with respect to hospital stay, need for admission to ICU or in-hospital mortality.
When a COPD patient sees their physician for an LRTI, it is important to distinguish between an exacerbation and pneumonia, because although pneumonia does not have a poorer prognosis as regards mortality or hospital stay,4 it is potentially more serious. Few studies have compared the characteristics of acute exacerbation and pneumonia13-17 in COPD patients, traditionally identified by radiological condensation. Recently, Huerta et al.13 showed that COPD exacerbation and pneumonia differed in clinical and inflammatory expression, with a greater increase in biological markers such as CRP and procalcitonin (PRL) in pneumonia, as well as a higher incidence of fever, chills, pleuritic pain and crepitus.
Our results confirm the findings of other authors. Most published studies have been conducted in small study populations13–16 with similar characteristics, with higher levels of obstruction in the ECOPD group13 and greater need for LTOT.13 Lieberman et al.14 showed that COPD patients admitted for pneumonia had higher rates of abrupt onset of symptoms, hypoxemia and fever during admission. To differentiate these cases from exacerbation, Huerta et al.13 also identified higher rates of fever, chills, pleuritic pain and crepitus on admission as symptoms consistent with pneumonia in COPD patients. This confirms the idea of pneumonia as an infectious disease with greater inflammatory involvement at systemic level (fever, hemodynamic change, more abrupt clinical course).
More recently, the usefulness of biological markers, particularly CRP and PRL, has been investigated in order to differentiate between COPD exacerbations and pneumonia, and to identify bacterial infections that could benefit from antibiotic treatment. CRP has been shown to be effective in distinguishing between exacerbation and pneumonia, and has proven useful as a prognostic factor. In the study by Justo et al.18 the CRP values on admission were higher in community-acquired pneumonia than in COPD exacerbations. This difference in CRP from admission to 24h after commencing treatment could be a useful diagnostic and prognostic tool. Similarly, PRL has also been shown to be useful as a marker of bacterial infection in the population with COPD and pneumonia.19 Several studies have recently been published by different groups recommending the use of biomarkers to reduce the use of antibiotics in patients with LRTI (pneumonia and ECOPD).20
The usefulness of biological markers in the differential diagnosis between ECOPD and PCOPD has been described by Huerta et al.13 where the latter is characterized by a greater increase in biomarkers, mainly during the first few days following admission. Other authors such as Jeong et al.16 have also demonstrated their usefulness.
According to arterial blood gas results at the time of admission, higher pCO2 values in the arterial blood gas in patients with ECOPD, a finding also reported by other authors,13,16 can be attributed to a greater need for LTOT.
With respect to other laboratory results, the hypoalbuminemia and hypoproteinemia observed in patients with ECOPD could be related to the findings of Schools et al.21 who noted malnutrition as one of several risk factors for pneumonia. In contrast, the increase in alpha-1-globulin levels could be related to greater systemic inflammation in episodes of PCOPD.
The microbiology of pneumonia in COPD has been widely studied. COPD patients usually have a higher percentage of H. influenzae and P. aeruginosa with respect to other microorganisms that are more common in non-COPD patients, such as L. pneumophila.22 Increased incidence of P. aeruginosa isolation in patients with pneumonia and underlying COPD might justify broadening empirical treatment in these patients, even without the presence of other risk factors for P. aeruginosa, such as the presence of bronchiectasia, admission to ICU, or need for mechanical ventilation.
Numerous studies have been published assessing the prognostic factors in COPD patients with pneumonia. Most of these have involved small cohorts and yielded inconsistent results, probably due to their heterogeneity. One meta-analysis concluded that mortality did not increase with the combination of COPD and pneumonia.4 According to a study by Rello et al.6 the higher mortality rate of pneumonia in patients with COPD was associated with bilateral pneumonia, bacteremia and shock, and with a higher incidence of P. aeruginosa.
Comparing patients with ECOPD and PCOPD, Lieberman et al.11 showed that a higher number of COPD patients hospitalized for pneumonia were admitted to the ICU, and had a greater need for mechanical ventilation, longer hospital stay and higher mortality.
Two limitations of our study are that the inclusion period coincided with a flu epidemic, and the study was conducted in a single center, which may limit the external validity of our results.
Another limitation, inherent to observational studies, is the clinical and radiological diagnosis of COPD exacerbations and pneumonia made by the medical team in routine clinical practice, although this was reviewed by one of the study investigators (Internal Medicine specialist).
In conclusion, we have observed that the clinical and laboratory characteristics and etiology of pneumonia in hospitalized COPD patients differ from those observed in exacerbation of COPD. Therefore, the parameters described above, in addition to conventional X-ray studies, may help in the differential diagnosis between ECOPD and PCOPD in patients presenting in the Emergency department for LRTI symptoms.
Authors’ Specific ContributionsRamon Boixeda helped in the study design, coordinating data collection, data analysis and interpretation, and in writing the article.
Sandra Bacca helped in the data collection and interpretation, and in writing the article.
Lorena Elias helped in the data collection and interpretation, and in writing the article.
Josep Anton Capdevila helped in the study design, data analysis and interpretation, and in writing the article.
Xavier Vilà helped in writing the article.
Montserrat Mauri helped in writing the article.
Jordi Almirall helped in the data interpretation and in writing the article.
FundingThere was no source of funding.
Conflict of InterestsThe authors declare that they have no conflict of interests.
We would like to thank the Department of Internal Medicine and the Respiratory Medicine Unit of Hospital de Mataró–Consorci Sanitari del Maresme for their assistance in conducting this study, and Agustí Viladot for his help in the literature search.
Please cite this article as: Boixeda R, Bacca S, Elias L, Capdevila JA, Vilà X, Mauri M, et al. La neumonía como comorbilidad en la enfermedad pulmonar obstructiva crónica (EPOC). Diferencias entre la exacerbación aguda de la EPOC y la neumonía en los pacientes con EPOC. Arch Bronconeumol. 2014;50:514-520.