Paraneoplastic syndromes are clinical manifestations of spontaneous antitumor immune responses against antigen proteins expressed on tumor cells. The most common paraneoplastic nephropathy associated with solid tumors is membranous nephropathy (MN), and the estimated prevalence of tumors in patients with MN is around 10%.1 And the majority of tumors related to MN are lung cancer.1,2
The advent of an immune checkpoint inhibitor dramatically changed the treatment of various tumors. In metastatic non-small cell lung cancer (NSCLC), the inhibitors of programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1) significantly prolonged overall survival compared with that of chemotherapy and has become a standard treatment.3–5 Whereas, the PD-1/PD-L1 inhibitors may also clinically manifest unique side-effect profiles caused by T cells’ action against self-antigens, known as immune-related adverse events (irAEs) and includes pneumonitis, colitis, rashes, hepatitis, pancreatitis, thyroiditis, and nephritis.6,7 In general, antitumor treatment is considered to improve paraneoplastic syndrome; however, little is known about the safety and efficacy of PD-1/PD-L1 inhibitors for patients with paraneoplastic syndrome. Here, we reported a patient treated using nivolumab, a PD-1 inhibitor, for NSCLC with paraneoplastic MN. A durable response to nivolumab was observed, without the exacerbation of nephrotic syndrome.
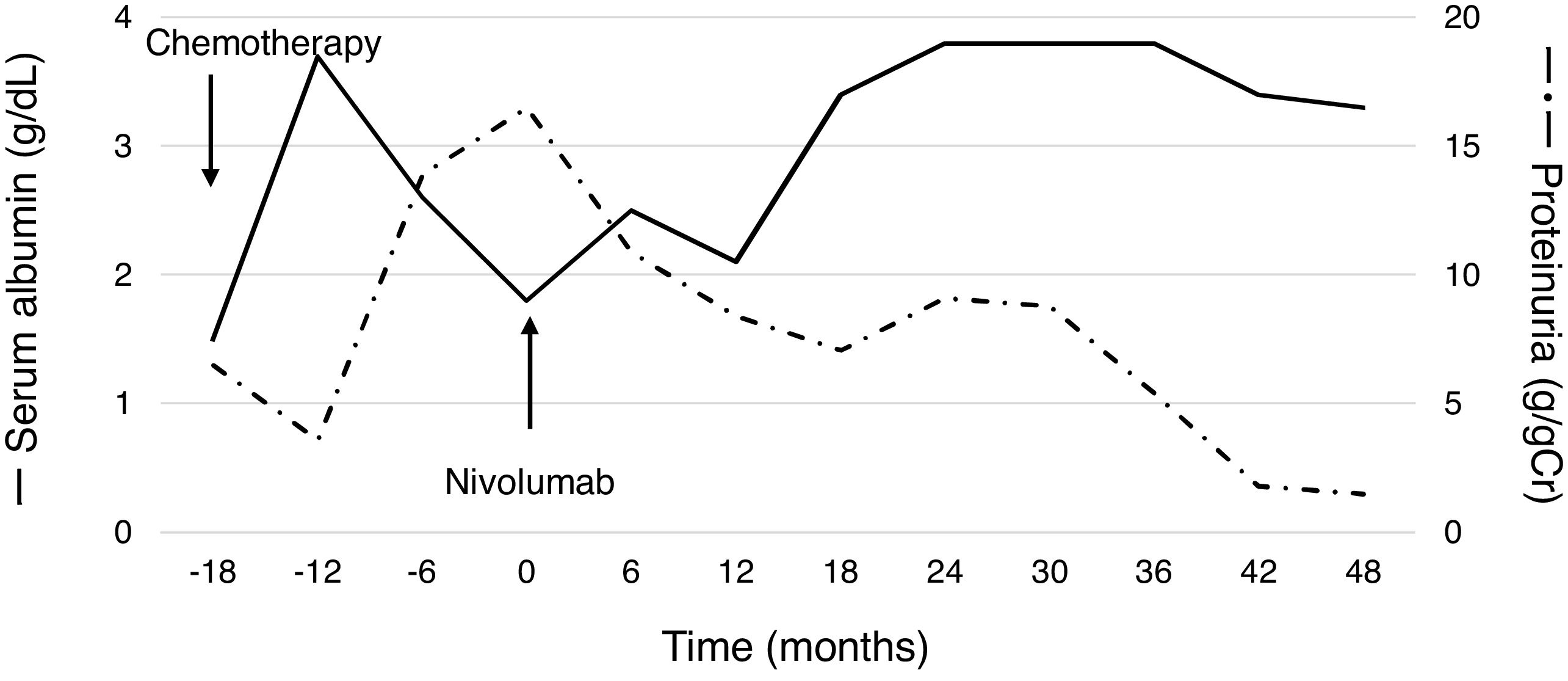
A 60-year-old man with peripheral edema due to hypoalbuminemia and proteinuria was diagnosed with nephrotic syndrome, and subsequent renal biopsy revealed MN. Although prednisolone and cyclophosphamide were administered, no improvement of nephrotic syndrome was observed. In addition, a nodule in the right upper lobe of the lung and multiple low-density areas in the liver on computed tomography developed during the treatment course. The biopsy samples from the lung demonstrated squamous cell carcinoma and he was diagnosed with stage IV non-small-cell lung cancer (NSCLC) harboring paraneoplastic MN. After the diagnosis of NSCLC, steroids and other immunosuppressive treatments were discontinued, whereas he received furosemide, lovastatin and candesartan for the management of nephrotic syndrome. Besides, cytotoxic chemotherapy of carboplatin plus S1 followed by docetaxel resulted in tumor regression accompanying the partial improvement of nephrotic syndrome; hypoalbuminemia and proteinuria improved (Fig. 1). After several months, however, nephrotic syndrome exacerbated and disease progression with metastatic lymph node enlargement also developed. At that time, nivolumab was approved for patients with previously treated NSCLC in Japan and he was treated using nivolumab. Thereafter, partial tumor response was achieved accompanying the partial remission of nephrotic syndrome (Fig. 1). The disease progressed four years after starting nivolumab, but paraneoplastic syndrome was not exacerbated during treatment.
One of the proposed mechanisms of paraneoplastic MN is the deposition of immune complexes formed by antibodies generated against an antigen identical to or an epitope similar to an endogenous podocyte antigen in the glomerular basement membrane beneath the podocytes.8 Therefore, the exacerbation of paraneoplastic syndrome due to heightened antitumor immune responses by PD-1/PD-L1 inhibitors directed against endogenous antigens was of concern.9 To date, there are no prospective studies of outcomes for solid tumors with paraneoplastic syndrome following PD-1/PD-L1 inhibitor treatment. Only one retrospective study demonstrated that neurological paraneoplastic syndrome in particular is exacerbated after treatment using PD-1/PD-L1 inhibitors. As such, physicians should carefully monitor patients with paraneoplastic syndromes.10 Our case demonstrated the safety and efficacy of nivolumab for NSCLC patients with paraneoplastic MN; however, further studies are required because lung cancer is frequently related to paraneoplastic syndrome. Moreover, future studies should focus on the detailed mechanisms and identification of patients at risk of exacerbated paraneoplastic syndrome.