Ehlers–Danlos syndromes (EDS) are a group of rare genetic disorders that affect connective tissue, characterized by joint hypermobility, skin hyperextensibility, and tissue fragility. The majority of the currently recognized 13 EDS subtypes1 are due to gene variants leading to impaired synthesis or structural abnormalities of different collagen types. Type IV or vascular EDS (EDS-IV) is an autosomal dominant multisystem disease caused by defects in type III collagen, encoded by the COL3A1 gene, in chromosome 2 (2q32.2).1 Type III collagen is a major constituent of the interstitial matrix in many tissues, including blood vessels. Accordingly, aneurysms and rupture of large arteries, spontaneous bowel perforation and uterine rupture during pregnancy are life-threatening complications of EDS-IV. EDS-IV is the most severe form of EDS, with most individuals dying before the 5th decade.1 Pulmonary involvement may occur without extrathoracic manifestations,2–6 and the diagnosis of such cases may be made only at post-mortem examination.6 Spontaneous pneumothorax or haemopneumothorax are the most frequent complications, but cavitary lesions, haemoptysis and pulmonary haemorrhage have also been reported.2–6
Herein, we report the occurrence of severe haemoptysis associated with massive pulmonary thrombosis, with a fatal outcome, in a young adult male with EDS-IV. The patient's diagnosis was established by genetic testing in mid-adolescence, as soon as the family's pathogenic COL3A1 (c.3517_3519del/p.(Gly1173del)) variant was identified. The patient had been free of significant EDS-related complications until the age of 17 years. The patient's father and one maternal aunt died from spontaneous aortic dissection/rupture, early in the 4th decade of life.
Our patient had a first hospital admission for haemoptysis at the age of 17. At the time, small areas of ground-glass opacities suggestive of alveolar haemorrhage were observed on chest computed tomography (CT). Over the next two years, he had occasional episodes of small-volume haemoptysis, often preceded by a tingling sensation in the head and neck.
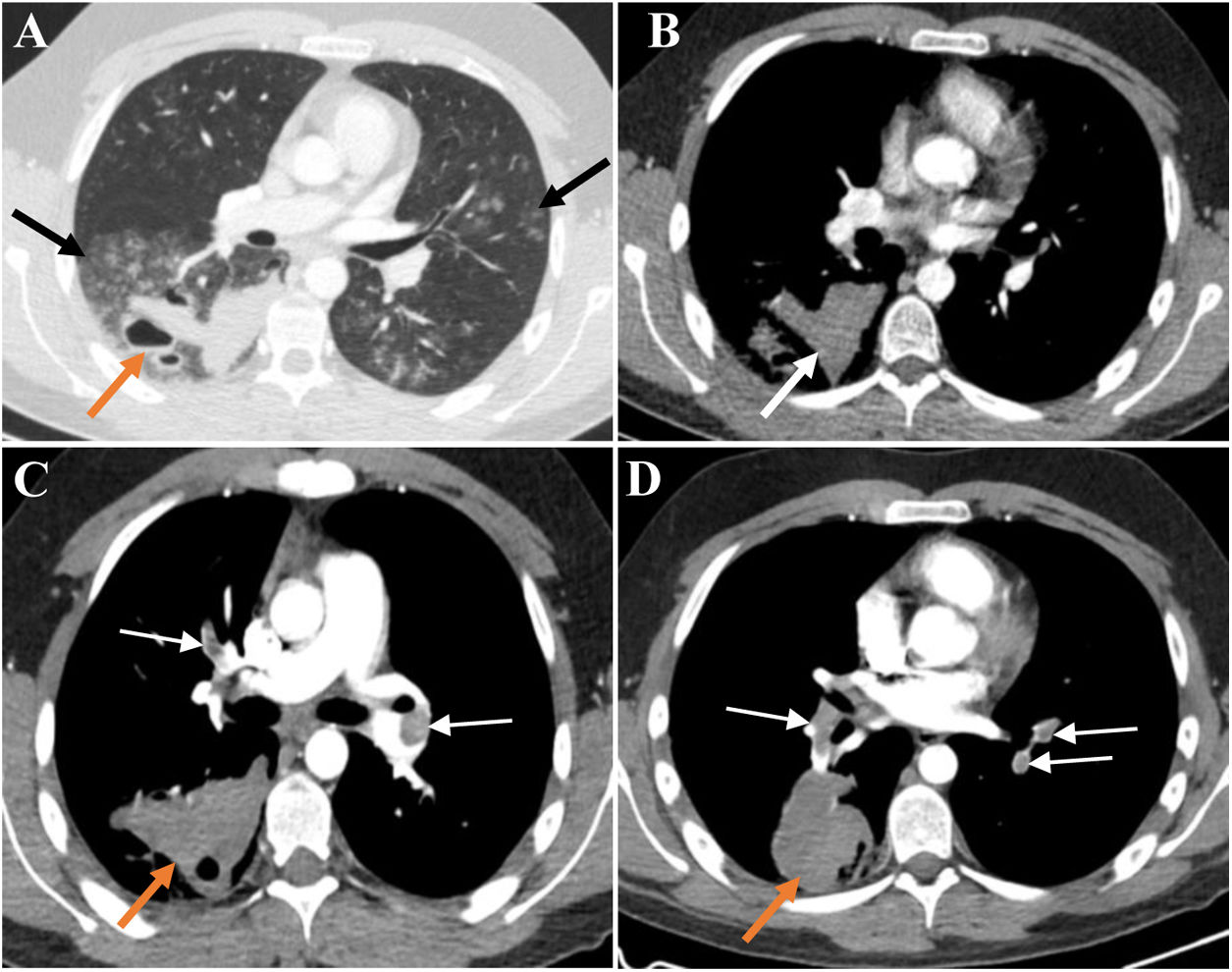
At age 19, the patient needed hospitalization for large-volume haemoptysis. In the previous days, he had intermittent episodes of small-volume haemoptysis preceded by what he described as a “clicking” sensation in the head and, right-sided paraesthesia and mild motor weakness. The chest CT scan (late arterial phase) showed multiple bilateral ground-glass opacities suggestive of diffuse alveolar haemorrhage and large fluid-filled cavities with air-fluid levels suggestive of haemorrhagic cavitation (Fig. 1A/B). Brain and cervical magnetic resonance (MR) imaging, MR angiography of the carotid and vertebrobasilar circulations, including the supra-aortic arches, and electroencephalogram were unremarkable. Despite the decrease in the haemoglobin level relative to baseline (respectively 15.3g/dL and 13.8g/dL), the patient remained hemodynamically stable without respiratory failure. Cough suppressive and antifibrinolytic therapies were initiated. Since the patient did not have any bronchial artery hypertrophy on CT scan and due to the high risk of vascular manipulation, bronchial arteriography was not performed. The surgical risk was also very high; therefore, the patient was not a candidate for thoracic surgery at that time.
(A) Late arterial phase chest CT depicts bilateral peribronchovascular groundglass opacities (GGO), especially in the right lower lobe (black arrows). In the right lower lobe thick-walled cavitary lesions with air-blood levels are also seen (orange arrow). The high density of blood-containing cavities is illustrated in B (white arrow). The GGO translate diffuse alveolar haemorrhage and the cavitary lesions result from the spontaneous rupture of the lung. There was no evidence of haemothorax or pneumothorax.(C and D) CT pulmonary angiographic study shows bilateral pulmonary thromboembolism (white arrows) and enlarged haemorrhagic cavity in the right lower lobe (orange arrows). No signs of pulmonary hypertension or right ventricular strain were present.
A pulmonary CT angiography obtained on day 7 of hospital stay showed extensive bilateral thrombosis and an enlargement of the haemorrhagic parenchymal cavitation, but no actively bleeding arteries (Fig. 1C/D). There were no signs of deep vein thrombosis on Doppler ultrasound examination of the lower limbs. Despite the striking radiological signs, the patient remained asymptomatic. Transthoracic echocardiogram did not show any signs of right ventricular dysfunction, and the Pulmonary Embolism Severity Index (PESI) score was 29 (class I), translating into a low mortality risk.7 Given the high bleeding risk and the low overall risk stratification, hypocoagulation was not prescribed.
Two days later, the patient was admitted to the intensive care unit, following a syncope episode with echocardiographic evidence of right ventricular dysfunction. Anticoagulation with unfractionated heparin and inotropic support were instituted. After about 24h of clinical stability, his haemodynamic condition suddenly deteriorated due to supraventricular tachycardia, followed by cardiac arrest that did not respond to advanced life support measures and thrombolysis. Autopsy was not performed.
To our knowledge, this is the first report of massive pulmonary thrombosis in a patient with EDS-IV seeking medical attention for recurrent haemoptysis. Arterial bleeding complications are typical of EDS-IV and several cases of severe haemoptysis, including with fatal outcomes, have been described.2,4,6 Platelet dysfunction may additionally aggravate the constitutional haemorrhagic propensity.8 Contrastingly, thrombotic events seem to be exceedingly rare in patients with EDS,9 but have been reported in EDS-IV10–12 and other EDS subtypes.9,13 In one of the EDS-IV cases, the patient had renal infarctions caused by emboli from a thrombotic renal artery aneurysm, and hypocoagulation was not prescribed either, with a favourable outcome.12
Major treatment decisions taken in our patient were based on validated risk stratification algorithms for pulmonary embolism/thrombosis7 and current clinical approaches to the management of haemoptysis.14 The delay in starting anticoagulation and the antifibrinolytic therapy might have facilitated the thrombotic event. Furthermore, haemostatic abnormalities were not screened, such as coexisting prothrombotic conditions or vitamin-D deficiency, which have been suggested as prognostic modulators in EDS-IV.8,11,15 However, taking into consideration the enlarging haemorrhagic cavitary lesion, the haemorrhagic propensity and the possibility of platelet dysfunction, the bleeding risk seemed to prevail when we decided not to initiate hypocoagulation therapy.
Despite the comprehensive neuroradiological and electroencephalographic evaluation, the aetiology of the neurological symptoms in close temporal association with the episodes of haemoptysis remained elusive, which is in line with previous observations in EDS-IV patients.3,4 In this case, a post-mortem examination could have been useful to characterize the lung pathology,4,6 as well as to elucidate the aetiology of the pulmonary embolism/thrombosis, but could not be carried out.
With this report, we aim to call the attention of respiratory physicians to this rare disorder, since haemoptysis, pneumothorax and pulmonary substance loss with cavitation can be the presenting manifestation of EDS-IV. Our patient illustrates the complexity of the pulmonary involvement in EDS-IV and the challenge of managing its haemorrhagic complications since the excessive vascular and tissue friability restricts diagnostic and therapeutic options. As in other rare, multisystem disorders, specific evidence-based treatment guidelines are not available, and patient care by multidisciplinary care teams is highly recommended.1