Paraneoplastic vasculitis (PNV) represents 2%–5% of all types of vasculitis and occurs in approximately 1 in 1800 hematological malignancies and 1 in 80800 solid tumors.1 To be considered PNV, both vasculitis and malignancy must be identified within a period of 12 months.2 The most common site for PNV is the skin, and almost half of all cases appear as leukocytoclastic vasculitis (LCV). We report the case of a woman who developed palpable purpura in the lower limbs that led to a diagnosis of lung cancer.
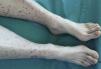
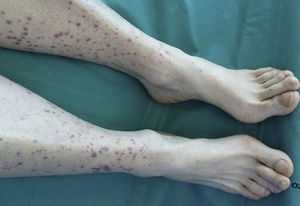
A 57-year-old woman, former smoker of 15 pack-years, cessation 22 years previously, history of left intraductal breast cancer at the age of 36 years, treated with breast-conserving surgery, chemotherapy and radiation therapy, with no signs of relapse on her last check-up 2 years previously. She was admitted to the hospital with a 10-day history of wrist and knee pain, associated with purpuric lesions on the lower limbs. In the last 72h, she had presented abdominal pain, vomiting and abundant liquid stools with no visible mucus, pus or blood. She reported a 6-month history of dry cough, anorexia-cachexia, and 4kg weight loss. On physical examination, she was seen to be asthenic, with a poor-to-middling general condition, body mass index 17.42, blood pressure 139/93, temperature 36.7°C. No significant lymphadenopathies were found on palpation, cardiopulmonary auscultation was normal. She had diffuse pain on palpation of the abdomen, which was soft, depressible and with no signs of peritoneal irritation. Musculoskeletal examination revealed palpable purpura on the lower legs, with some lesions on the thighs, nail clubbing in both the fingers and toes (Fig. 1), and no pain, joint limitation or synovitis. Clinical laboratory test results showed: Hb 12.7g/dl, Hct 37.4%, MCV 101.9, MHC 34.6pg. All biochemistry parameters, immunoglobulins, C3, C4, ANA, ANCA, anticardiolipin antibodies, lupus anticoagulant, cryoglobulins, β2-microglobulin, tumor markers (α-fetoprotein, CEA, CA-125, CYFRA 21.1, enolase), and HCV, HBV and HIV serologies were normal or negative. ESR 69. CRP 10.4mg/dl. Urinanalyis and coagulation studies were normal, with the exception of fibrinogen 619mg/dl. Proteinuria: 0.4g/24h, that later normalized; standard urine testing and sediment studies were normal. Skin biopsy: small vessel leukocytoclastic vasculitis. Chest X-ray: left upper lobe (LUL) nodule. Thoracoabdominal computed tomography: 25mm left suprahilar mass with consolidation in the adjacent parenchyma extending to the chest wall and causing LUL atelectasis. Fiberoptic bronchoscopy: mass in LUL causing stenosis of the left upper lobe bronchus. Selective bronchial aspiration and biopsy: well-differentiated epidermoid carcinoma. Lung function tests: normal. PET: left suprahilar mass 41mm×49mm×48mm, SUVm 20.7, adjacent parenchymal condensation 31mm×21mm×28mm, extending to the left anterior chest wall, SUVm 11.7. Focal deposit at the level of the third anterior rib, 11mm×11mm×16mm, SUVm 6.2. The patient received serum and prednisone, with good clinical progress and resolution of abdominal symptoms and skin lesions within 10 days. The tumor was confirmed by the Thoracic Surgery department to be inoperable, so chemotherapy for epidermoid carcinoma of the lung, cT4N0M0, began in the Oncology department.
Around 50%–60% of paraneoplastic cutaneous vasculitis is LCV, and 15% is Schönlein-Henoch purpura (SHP).1 Loricera et al.2 published one of the largest series of cutaneous PNV, consisting of 421 adults with cutaneous vasculitis, of which only 16 (3.8%) were paraneoplastic, 7 associated with solid tumors (lung adenocarcinoma) and 9 with hematological cancers. Palpable purpura occurred in 15 patients, 4 of whom had arthralgia and/or arthritis and 2 abdominal pain. Mean age was 67 years and delay before reaching a cancer diagnosis was 17 days. Histology in all cases was LCV. Solans-Laqué et al.3 reported a series of 596 cases of vasculitis over a period of 15 years. They found 15 PNVs associated with solid tumors (2.5%): 9 LCV, 2 SHP, 1 polyarteritis nodosa, and 3 cases of giant cell arteritis. In some publications, PNV meet criteria, either clinically or due to IgA deposits in biopsies, for a diagnosis of SHP. Zurada et al.4 presented 3 cases of paraneoplastic SHP and reviewed 31 cases published to date, of which 61% were associated with solid tumors (8 lung), and 39% hematological. Half of all SHP cases appeared within 1 month of tumor diagnosis or metastasis. More recently, Zhang et al.5 reviewed 13 previously published cases of SHP associated with lung cancer: 8 epidermoid, 3 adenocarcimonas and 2 small cell cancers. Six occurred simultaneously with the tumor, 6 preceded it, and 1 appeared subsequently.
We can conclude, then, that while PNV is an uncommon manifestation, it can be an initial presentation of tumor disease. Moreover, in cases of persistent or chronic vasculitis that does not respond well to treatment, particularly in elderly patients, paraneoplastic syndrome must be ruled out. Development or relapse of vasculitis in a cancer patient should raise the suspicion of tumor recurrence.3
Please cite this article as: Pantoja Zarza L, Díez Morrondo C, Castro Rodríguez E. Vasculitis cutánea paraneoplásica asociada a cáncer de pulmón. Arch Bronconeumol. 2015;51:365–366.