Oxygen therapy, like all technology-based treatments, is continuously evolving. There are no doubts as to its effectiveness in the treatment of acute and respiratory failure in different clinical scenarios. However, the dosing guidelines for oxygen therapy are not as strict as for other treatments. The use of higher than necessary flows over excessively long periods, derived from the clinician's perception of it as a ‘life-saving treatment with few side effects’, has led to a rather liberal use of this intervention, despite evidence that overuse and suboptimal adjustment can be harmful. The titration of oxygen therapy, which is traditionally performed manually, has been shown to be beneficial. Recently, new devices have been developed that automatically adjust oxygen flow rates to the needs of each patient, in order to maintain stable oxygen saturation levels. These closed-loop oxygen supply systems can potentially reduce medical error, improve morbidity and mortality, and reduce care costs. Familiarizing the medical community with these technological advances will improve awareness of the risks of the inappropriate use of oxygen therapy. The aim of this paper is to provide an update of recent developments in oxygen therapy titration.
La oxigenoterapia, como todos los tratamientos que dependen de la tecnología, no para de evolucionar. No hay dudas respecto a su eficacia en el tratamiento de la insuficiencia respiratoria aguda y crónica en diferentes escenarios clínicos. Sin embargo, su dosificación terapéutica no es tan estricta como la de otros tratamientos. El uso de flujos más altos de lo necesario y por periodos demasiado prolongados, derivados de la percepción de «terapia salvavidas con pocos efectos secundarios» por parte del clínico, ha conllevado un uso bastante liberal de este medicamento, pese a la evidencia de que tanto su uso en exceso como su ajuste subóptimo tienen efectos nocivos. La titulación de la oxigenoterapia ha demostrado efectos beneficiosos en los pacientes. Tradicionalmente, se ha realizado de forma manual. Recientemente, se han desarrollado nuevos dispositivos para ajustar automáticamente las tasas de flujo de oxígeno a las necesidades de cada paciente, con el objetivo de mantener las saturaciones de oxígeno estables. Estos sistemas de suministro de oxígeno de circuito cerrado tienen el potencial de reducir el error médico, mejorar la morbimortalidad y reducir los costes del cuidado. Dar a conocer estos nuevos avances tecnológicos ayudará a concienciar a la comunidad médica de los riesgos del uso inadecuado del tratamiento con oxígeno. Este trabajo pretende ser una puesta al día de las recientes novedades en relación con la titulación de la oxigenoterapia.
Oxygen therapy, like all technology-based treatments, is continuously evolving. It is defined as the therapeutic use of oxygen (O2) administered at concentrations higher than those of room air to treat or prevent the effects of hypoxemia.1–3 The aim of administering oxygen is to maintain oxygenation levels above the range of respiratory failure (O2 blood pressure [PaO2]>60mmHg, arterial oxygen saturation measured by pulse oximetry [SpO2]>90%). It is widely used in different care areas in patients with acute and chronic respiratory problems, at home and in the hospital.4 Although O2 is considered a medication by health authorities, its widespread use and image as a benign, uncomplicated treatment have resulted in careless dosing and little concern over side effects, in spite of the available scientific evidence.5,6 As a medication, O2 should be used with the same rigor as any other drug, and indications and dosage must be evidence-based. Only in this way will we achieve the main objectives of this therapy: improved survival, proper correction of hypoxemia, and avoidance of the risks of excessive oxygenation. In this review, we will address the need for oxygen titration, the advantages of automatic and manual titration, the required features of automatic titration devices, the devices available on the market and the evidence supporting them, and, finally, the limitations and shortcomings of existing equipment.
Why Oxygen Therapy Titration is NecessaryBoth hypoxemia and hyperoxia resulting from exposure to excessive O2 flows can have serious consequences for patients,7,8 as demonstrated by data from children and adults with acute and chronic respiratory failure.
There is ample clinical evidence of the undesirable effects of oxygen therapy in the neonatal population. An insufficient supply of O2 has deleterious effects on neurological development and pulmonary circulation,9–15 while an excessive supply is associated with systemic oxidative damage in different organs.16–19 For this reason, maintaining the right oxygenation levels is essential. Traditionally, oxygen titration was performed manually, by adjusting O2 flows to keep SpO2 within the desired range. However, children titrated manually have shown SpO2 levels below the range for more than 50% of the time and above the range for at least 33% of the time. This could be related to both the ability of health personnel to respond to fluctuations in SpO2, and the excessive tolerance of children to high levels of SpO2.20–22
In adults, the goal of oxygen therapy is also to avoid the consequences of hypoxemia, but the well-known harmful effects of hyperoxia are, if anything, even more frequently neglected.23
In cases of acute respiratory failure, hypoxemia has been associated with an increase in mortality following myocardial infarction and serious head injuries.24–26 It is also associated with increased mortality in patients admitted to intensive care units (ICU) and a greater number of complications in the postoperative period.27,28 Suboptimal use of oxygen therapy has also been observed in patients with chronic obstructive pulmonary disease (COPD) exacerbations, in whom the effects of hypoxemia are particularly significant and widely known.29,30 These patients are sometimes poorly oxygenated over long periods.31
Hyperoxia also entails a greater risk for patients with acute respiratory failure, not because the risk is unknown, but because it is minimized by healthcare professionals.6,7 Its presence is associated, irrespective of hypoxemia, with an increased risk of death after cardiorespiratory arrest and increased mortality in patients with serious head injuries.25,32,33 Nearly 25% of patients with COPD exacerbations are exposed to significant periods of hyperoxia, which is related to higher mortality and worse clinical outcomes that can be prevented by careful adjustment of O2 flows.34–36 In spite of all this evidence, the clinical reality is that the vast majority of patients receive high-flow O2 during COPD exacerbations, an intervention that carries an increased risk of respiratory acidosis.37
Patients with stable chronic respiratory failure often present long-term hypoxemia, this being the main reason for reduced exercise tolerance and multiple, well-characterized complications (pulmonary hypertension, right heart failure, polycythemia, and increased mortality).38,39 The use of continuous home oxygen therapy (CHOT) in COPD patients has shown beneficial effects on survival, quality of life, and exercise tolerance,2,40–42 and its indication has been extended, by analogy, to other chronic lung diseases.43–47 In CHOT, oxygen flow is usually fixed. This is not always optimal,48 since the needs of patients vary throughout the day. More than half have O2 desaturation during their usual activities.49,50
All these data show that oxygen therapy must be adapted to individual patient requirements. The available evidence indicates that the proper use of oxygen therapy is an important factor in clinical outcomes,51 and that a prescription based on continuous monitoring can significantly increase the percentage of time with an appropriate SpO2, while decreasing the amount of O2 prescribed.52 Essential objectives in the adjustment of oxygen therapy should be:
- •
To optimize therapy and safety, minimizing the number of episodes of hypoxemia and hyperoxia. The prescription should be aimed at achieving a saturation range, rather than establishing high O2 flows, regardless of arterial O2 levels.35
- •
To tailor O2 flow to the real needs of patients in their daily activities. O2 flow selection does not always meet the needs of the patient, and may be insufficient when demand increases.53
- •
To optimize the consumption of O2 to increase time on portable devices; the technological limitations of these devices underline the need for measures that adapt therapy to real life.
- •
To improve morbidity and mortality outcomes.
- •
To reduce the excessive costs generated by inappropriate use.
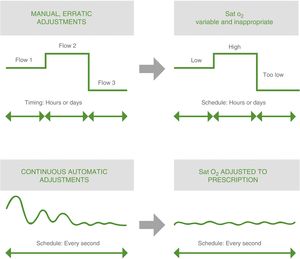
The current situation shows that these goals are difficult to achieve in clinical practice.54 As already mentioned, the traditional method of manual titration has significant limitations. Trained personnel are needed both in the hospital and at home. Healthcare personnel in ICUs dedicate a significant amount of a time to titration, and the process may be burdensome for patients, and can contribute to poor therapeutic compliance. Moreover, the adjustment achieved is suboptimal and episodes of under- and overdosing are common, decreasing the efficiency of the treatment and jeopardizing patient safety.
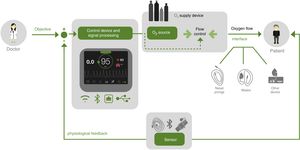
Automatic Oxygen TitrationAll these findings, concerns, and limitations have led in recent years to the development of physiological, controlled, closed-circuit medical devices for the automatic titration of O2 flows. These are generally defined as devices that use 1 or several sensors to automatically change a physiological variable (O2 flow), thus maintaining the oxygenation level determined for each patient55 (Fig. 1). Automatic O2 titration consists of 3 components: a system for monitoring oxygenation; an algorithm that determines the O2 flow settings to achieve the appropriate oxygenation level; and an O2 source.53
- 1)
Monitoring oxygenation levels. Various systems can be used, depending on the variable selected. The use of permanent PaO2 electrodes and transcutaneous O2 pressure (PtcO2) electrodes is limited due to the need for an invasive catheter in the first case and frequent calibrations in the second. SpO2, as a noninvasive method that does not require calibration, has thus become the preferred monitoring system.
- 2)
Algorithms. Algorithms that determine O2 flow settings required to maintain established SpO2 figures compare continuous or intermittent SpO2 results with target levels and adjust O2 flow accordingly. A major challenge is to determine when hypoxemia is a real event and when it is due to movement artifacts or a weak pulse oximeter signal, in order to avoid the risk of O2 overdosing and hyperoxia. Moreover, the response to hypoxemia should be sufficient to correct the situation, but without producing rebound hyperoxia. Algorithms are specific to each device and hard to compare, so they must all be validated clinically before use.
- 3)
Source of O2. O2 can come from a wall outlet or a bottle of compressed O2, or from any other source supplying a flow that can be controlled by an external mechanism. The O2-enriched gas can then be administered to the patient via mechanical ventilators, continuous positive airway pressure (CPAP) devices, high-flow equipment or oxygen therapy devices, such as nasal prongs. Venturi masks are not appropriate for O2 automatic titration, as the fraction of inspired O2 (FiO2) will depend on the size of the mask aperture, and not on the flow supplied. Ventilators and high-flow equipment must have accurate, robust mixers that can withstand the required changes in O2 flow.
The mode of operation is easy and intuitive. The pulse oximetry signal is analyzed on a continuous basis by the central unit, which increases or decreases the flow of O2 as needed to maintain the predetermined level of SpO2.
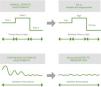
These devices have the potential to optimize oxygen therapy by reducing the risk of over- and underdosing and overburdening caregivers, and improving patients’ clinical outcomes. Compared to the erratic adjustment of manual titration, these devices can achieve appropriate, sustained oxygenation levels by continuous, automated adjustment of the O2 flow53 (Fig. 2). The results of a recent meta-analysis show how these devices increase the time during which SpO2 is maintained within the range predefined by the clinician compared with manual titration,56 opening up new possibilities in the area of oxygen therapy titration: automatic titration or automated titration.
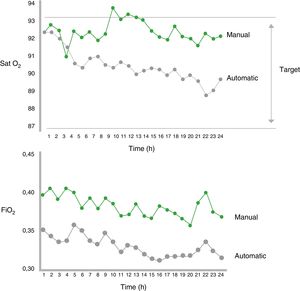
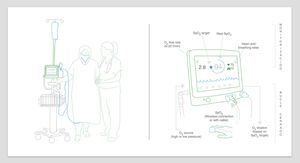
Clinical Data on Oxygen Therapy Automatic TitrationStudies in NeonatesThe first studies on automatic titration were conducted in neonates receiving mechanical ventilation or CPAP in a critical care setting. These studies assessed the effectiveness of automatic titration systems compared to manual titration (standard or performed by dedicated staff), analyzing the maintenance of oxygenation within the predetermined SpO2 range.57–63 Automatic titration systems were more effective than manual titration for controlling SpO2 and periods of hyperoxia were shorter62,63 (Fig. 3). Moreover, a significant decrease in the workload of staff was observed. Table 1 shows the characteristics of the most important studies in neonates.
Characteristics of Major Studies in Neonates.
| Studies in Neonates | % Time Within Range | % Time Above Range | % Time Below Range | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author | Year | Support | Monitor | Objective | Manual Mode | Automatic Titration | Manual Titration | Automatic Titration | Manual Titration | Automatic Titration | Manual Titration |
| Morozoff57 | 1992 | MV | SpO2 | 92% | Routine | Decrease in SD of 6/8 | NA | 23 | 39 | 27 | 32 |
| Sun58 | 1997 | MV | SpO2 | Set ±3% | Routine | 72 | 58 | NA | NA | NA | NA |
| Claure60 | 2001 | MV | SpO2 | 88%–96% | Dedicated staff | 75 | 66 | 10 | 15 | 17 | 19 |
| Urschitz61 | 2004 | CPAP | SpO2 | 87%–96% | Routine | 91 | 82 | ∼1.3a | ∼4.9a | ∼3.2a | ∼6.7a |
| Dedicated staff | 91 | ∼1.8a | ∼3.9a | ||||||||
| Morozoff59 | 2009 | MV | SpO2 | 90%–96% | Routine | 73 | 57 | ||||
| Claure62 | 2009 | MV | SpO2 | 88%–95% | Routine | 58 | 42 | 9.3 | 31 | 33 | 27 |
| Dedicated staff | NA | 16 | 4.6 | 6.6 | |||||||
| Claure63 | 2011 | MV | SpO2 | 87%–93% | Routine | 40 | 32 | 21 | 37 | 32 | 23 |
| 75%–98% | NA | NA | 0.7 | 5.6 | 4.7 | 5.4 | |||||
Routine manual mode: FiO2 monitored according to standard care.
Manual mode with dedicated staff: nursing staff devoted full time to FiO2 monitoring.
CPAP: continuous positive airway pressure; MV: mechanical ventilation; NA: not available; SD: standard deviation; SpO2: arterial oxygen saturation by pulse oximetry.
The first studies in adults were also conducted in critical care units, the first area in which this technology was applied, and usually in ventilated patients.64 In the last 10 years, research has targeted the development of portable devices that can also be used in less severe patients in other hospital areas, such as wards and the emergency department, and even outside the hospital setting. Automatic titration systems have been compared with conventional modes of O2 administration in COPD patients receiving CHOT during their normal activities65 and during exercise,66 and in healthy volunteers with induced hypoxemia.48 Automatic titration devices obtained better results than conventional manual titration or continuous fixed-flow O2 supplementation in terms of time within the SpO2 range and time of hypoxia or hyperoxia, generating significant O2 cost savings. A summary of the most important studies in adults is shown in Table 2.
Characteristics of Major Studies in Adults.
| Studies in Adults | % Time Within Range | % Time Below Range | % Time Above Range | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Author | Year | Support | Application | Range | Automatic Titration | Manual Titration | Automatic Titration | Manual Titration | Automatic Titration | Manual Titration |
| Johannigman64 | 2009 | MV | Intensive care | SpO2 92%–96% | 83 | 33 | 1.0 | 1.9 | 16.6 | 60.1 |
| Rice65 | 2011 | Cannula with oxygen sparing system | Domiciliary | SpO2≥90% | 71 | 41 | 22 | 23.5 | 7 | 35 |
| Continuous flow oxygen cannula | 50 | 22 | 28 | |||||||
| Cirio66 | 2011 | Oxygen cannula | Exercise | SpO2 94% | NA | NA | 19 | 38 | 19 | 38 |
| Lellouche48 | 2012 | Continuous flow oxygen cannula | Induced hypoxia | SpO2 92%–96% | 66.5 | 36.8 | 17 | 27.3 | 14.5 | 39 |
| Cannula with air | 26 | 67 | 4.1 | |||||||
| Lellouche71 | 2016 | Oxygen cannula | Hospital | Predefined by the clinician for each patient (∼90%) | 81.2 | 51.3 | 0.2 | 2.3 | 1.5 | 10.4 |
| Lellouche68 | 2016 | Oxygen cannula | Exercise | SpO2 92%–96% | 62.3 | 42.9 | 21.4 | 30.4 | 14.4 | 26.7 |
| Cannula with air | 41.6 | 51.4 | 6.9 | |||||||
| L’Her73 | 2017 | Oxygen cannula | Emergency room | SpO2 92%–96% | 81.3 | 51.8 | 3.2 | 5.1 | 4.2 | 21.6 |
| Huynh74 | 2017 | Oxygen cannula | Hospital | SpO2 90%–92% | NA | NA | 0.4 | 4 | 22 | 57 |
| SpO2 90%–97% | NA | 1.2 | 30 | |||||||
MV: mechanical ventilation; NA: not available; SpO2: arterial oxygen saturation by pulse oximetry.
There are currently only 3 validated portable devices marketed for adults: the Optisat AccuO2® (Medical, Minneapolis, USA) (Fig. 4); the O2 Flow Regulator® (Dima Italia Srl, Bologna, Italy (Fig. 5); and the FreeO2® (Oxynov, Quebec, Canada) (Fig. 6).
- a)
O2 Flow Regulator®. This device was validated by Cirio and Nava66 in COPD patients receiving long-term oxygen therapy. It adjusts O2 during exercise to maintain 94% SpO2. Patients performed 2 cycling exercises for a period of 15min with a constant load. The authors showed that patients had better oxygenation using the automatic titration system, thus reducing the workload of respiratory therapists. There are 2 models on the market, which can regulate a maximum flow of 10l/min (model 106A) and 20l/min (model 106A Plus), respectively.
- b)
AccuO2®. This device was also validated by Rice et al.65 in COPD patients. The authors randomly assigned 28 COPD patients receiving CHOT to 3 O2 management systems (continuous flow, O2-conserving device CR-50®, and AccuO2®) 8h a day, for 2 consecutive days at home. Twenty-two patients completed all 3 study arms. Compared with continuous flow O2 or the CR-50®, the AccuO2® maintained SpO2 values closer to the target. SpO2 variations were also lower with the AccuO2®, and the reduction in the consumption of O2 was even greater than with the CR-50®.
- c)
FreeO2®. This is the device for which most scientific evidence is available. It is distributed in Spain by Linde Médica. The model available on the market takes O2 directly from the wall and can reach flows of up to 20l/min. A new model with capacity to achieve flows of 50l/min will soon be released. It has 3 operating modes: neonates (maximum 5l/min), children (up to 10l/min), and adults (maximum 20l/min). The clinician selects the desired SpO2 on the monitor, limiting maximum flow, if necessary. The equipment will steadily regulate the flow, increasing or reducing it to maintain the preset SpO2. In the event of signal loss, the equipment maintains the flow delivered prior to the loss, as a safety mechanism. It can also function with continuous flow or simply be used as a pulse oximeter. Features include SpO2, flow, and heart rate alarms. The screen displays real-time data and trends of up to 72h.
The FreeO2® was originally validated by Lellouche and L’Her48 in a pilot study in 10 healthy volunteers with induced moderate hypoxemia. The subjects were randomized to receive air at a continuous flow of 1.5l/min, O2 at a continuous flow of 1.5l/min, or automatic titration with FreeO2®. The FreeO2® system was more efficient at maintaining target SpO2, and significantly decreased the rate of severe hypoxemia and hyperoxia, with lower O2 consumption than in the continuous flow arm.
The authors subsequently validated this device in different clinical situations.67 In an evaluation of the effect of the FreeO2® device in patients with moderate COPD without criteria for CHOT who experienced desaturation during exercise (endurance shuttle test), this system achieved better oxygenation (preset SpO2 range of 92%–96%) with fewer episodes of desaturation (SpO2<88%) than with a constant O2 flow at 2l/min and compressed air.68 An improvement in exercise tolerance was achieved with both automatic titration and constant flow O2, and although the distance covered improved by 17% with FreeO2®, the difference was not statistically significant. Moreover, higher than usual O2 flows were required to achieve these results with the FreeO2® (>5l/min in the majority of patients, and up to 10l/min in one subject). This raises questions about its use in clinical practice, due to the risk of associated hypercapnia (not observed in the study due to the limited exposure to hyperoxia), and also to the lack of devices capable of supplying these flows in existing portable oxygenation systems.
In a study of hypercapnic COPD patients receiving CHOT, Vivodtzev et al.69 showed that automatic titration with FreeO2® during a walk test was associated with a significant improvement in SpO2 without associated hypercapnia, but the distance walked in meters did not increase significantly. These results, combined with those of the LTOT study,70 in which the authors concluded that the use of supplementary O2 does not provide any clinically significant benefit in patients with moderate desaturation at rest or during exercise, show the need for further research to settle the many questions that remain to be answered in the field of portable oxygen therapy.
The Canadian group validated the FreeO2® device in patients with COPD exacerbations in two situations: at the time of admission to a hospital ward,70 and on arrival in the emergency department.71 In the first scenario, 50 patients were randomized to manual titration or FreeO2®. The authors concluded that the FreeO2® device was more effective at maintaining SpO2 at the programmed level than manual titration, and also reduced periods of desaturation and hyperoxia. Nurses and doctors also considered it an acceptable system. They did not find, however, significant differences in the average length of stay, admissions to the ICU, or the rate of readmissions.71 The authors also stated that this technology could significantly improve the efficiency of the health system by reducing costs by 20.7% per patient, calculated on a period of 180 days.72 In the second scenario, 187 patients with COPD exacerbation and need for O2≥3l/min were randomized to FreeO2® or manual titration upon arrival in the emergency room. The authors concluded that patients using the FreeO2® had better SpO2 control, with shorter times of hypoxemia and hyperoxia; they needed O2 for less time during their hospital stay and a higher percentage were weaned from O2, which reduced the time of oxygenation and improved the safety of the procedure. The device was well accepted by physicians, and no differences were observed in terms of early discontinuation of treatment.73 These authors are exploring other novel lines of research with this device in patients with acute coronary syndrome and in the management of central apneas, although the results are still unknown.74,75
Limitations of Automatic TitrationDespite the numerous studies on automatic titration, many questions remain. Do automatic titration devices work with any interface? Are the oximetry sensors sufficiently sensitive and reliable? Is there a risk of hardware malfunction due to development defects or electrical problems? What is their role in oxygen therapy? In the acute patient? In home therapy? During exertion?
In most studies, the circuit feedback variable was SpO2, and patients were continuously connected to a pulse oximeter. This is the only source of information that feeds the oxygen adjustment algorithm. In manual titration, however, the doctor has several sources of information (monitors, patient response, etc.), thus increasing patient safety. In this regard, the instability of the sensor to movements or physiological delay in the measurements is significant, so improvements in pulse oximetry will be key in the development of clinically effective monitors.
Moreover, the effectiveness of oximetry in assessing hyperoxia is very limited, as is its ability to promptly detect respiratory depression, even when is associated with significant CO2 retention. New lines of research, such as the oxygen reserve index (ORI), aimed at improving the reliability of measurements are of considerable interest.76 ORI is a non-invasive continuous variable provided by a generation of pulse oximeters that use multi-wavelength pulse co-oximetry. The ORI reflects oxygenation in the moderate hyperoxic range. This provides an early warning of deteriorated oxygenation well before any changes occur in SpO2, and is therefore a good complement to pulse oximetry the management of patients with oxygen therapy.76
Another problem of automatic titration is that an increase in O2 flow may not be an appropriate response when hypoxemia is due to hypoventilation. Moreover, to minimize the risk of masking continued deterioration in respiratory function by automatically increasing in O2 flow, automatic titration devices should warn of persistent increases in the O2 flow, even if SpO2 remains within the range.
Final ThoughtsIn conclusion, automatic titration systems improve the dosage of oxygen therapy while avoiding the deleterious consequences of hypoxemia and hyperoxia in acute and chronic respiratory failure. However, they cannot replace clinical monitoring, particularly in light of their limitations in correcting hypoventilation-induced hypoxemia. Despite these drawbacks, the clinical potential of these devices is considerable, and they may help doctors tailor oxygen therapy to the needs of individual patients. Let us not forget that, although oxygen therapy saves lives, when used inappropriately it can cause serious damage. Technological progress in this field will, without doubt, lead to the development more reliable equipment with more sensitive sensors with a capacity for faster response; however, new avenues of research in this area of knowledge are still needed. At the present time, automatic titration of oxygen therapy must remain a subject of research.
Conflict of InterestsSagrario Mayoralas Alises is the medical director of Oximesa Grupo Praxair, supplier of domiciliary respiratory therapies. Oximesa does not sell any of the devices described in the manuscript. The other authors state that they have no conflict of interests with regard to this manuscript.
Please cite this article as: Mayoralas-Alises S, Carratalá JM, Díaz-Lobato S. Nuevas perspectivas en la titulación de la oxigenoterapia: ¿es la titulación automática el futuro? Arch Bronconeumol. 2019;55:319–327.