Determinants of chronic obstructive pulmonary disease (COPD) in the early stages of its natural history are not well known. Improving our knowledge of these factors will help to design interventions that can modify prognosis.
Study objectives are: (a) to characterize a COPD population of young adults aged 35–50 years from a multidimensional point of view; (b) to compare these patients with smokers with normal lung function; and (c) to create a cohort of young adults aged 35–50 years (smokers or former smokers), with and without COPD, who will be followed in the future to improve understanding of the natural history of the disease.
Participants and MethodThis is a case–control multicenter study aimed at establishing a well-characterized cohort of young adults, smokers or former-smokers, with and without COPD, for subsequent follow-up.
A total of 311 participants (101 cases and 210 controls) were selected from approximately 30 primary care settings and 12 hospitals in 8 Spanish regions. Subjects were smokers or former smokers (>10 pack-years) aged 35–50 years. Diagnosis of COPD was based on a post-bronchodilator result of FEV1/FVC<70%.
The main study variables were: questionnaires on health, symptoms, exacerbations and daily physical activity, lung function tests, blood and sputum samples, and low-dose computed tomography. In the statistical analysis, COPD patient characteristics will be described and compared with control subjects using a logistic regression analysis.
Los determinantes en fases iniciales de la historia natural de la enfermedad pulmonar obstructiva crónica (EPOC) son poco conocidos. Entenderlos mejor es de capital importancia para poder diseñar intervenciones dirigidas a modificar su pronóstico. Los principales objetivos del estudio son: a) caracterizar a una población de adultos jóvenes con EPOC de forma multidimensional; b) comparar estos pacientes con sujetos fumadores con función pulmonar normal; y c) establecer una cohorte de adultos jóvenes con y sin EPOC, que pueda ser seguida a largo plazo para conocer mejor la historia natural de la enfermedad.
Participantes y métodoEARLY COPD es un estudio multicéntrico de casos y controles que permitirá establecer una cohorte de sujetos para su seguimiento posterior. Se seleccionaron 311 (101 casos y 210 controles) participantes reclutados en una treintena de centros de atención primaria y 12 hospitales de 8 comunidades autónomas españolas. Los participantes eran fumadores o exfumadores (>10 paquetes año) de entre 35–50 años de edad. Los casos presentaban una espirometría obstructiva con un FEV1/FVC<70% y los controles una espirometría normal con un FEV1/FVC≥70%. Las principales variables de estudio que se han determinado son las siguientes: cuestionarios de salud, síntomas, exacerbaciones y actividad física, pruebas de función respiratoria, análisis biológicos de sangre y esputo y TAC de baja radiación. Para el análisis estadístico de los resultados se describirán las características de los pacientes con EPOC y se compararán con los sujetos del grupo control mediante un modelo de regresión logística.
Chronic obstructive pulmonary disease (COPD) is a respiratory disease characterized by persistent symptoms and chronic airflow limitation, caused mainly by tobacco.1 It has a significant impact on patients’ quality of life and generates a high socioeconomic burden for the healthcare system,2 underlining the need for strategies for prevention and early treatment. However, due to the heterogeneous nature of the disease3 and the fact that it is diagnosed in advanced stages, knowledge of its pathology and biology is very limited.4
The current understanding of the natural history of COPD is based on the studies of Fletcher and Peto on lung function among British workers of different age groups, published in 1977.5 These studies showed an accelerated decline in forced expiratory volume in 1 second (FEV1) in the group of “susceptible smokers”. However, the heterogeneous nature of COPD has led to suggestions that different natural histories can exist within COPD that display varying etiopathogenic mechanisms.6,7 In fact, the results of the ECLIPSE study have shown that the accelerated decline in lung function mentioned above is absent in up to 60% of patients diagnosed and treated for COPD.8 There are also other unanswered questions about the natural history of the disease, such as the effect of lung development in childhood and adolescence, disease progression in the early stages, the role of bronchial hyperreactivity and infections, the influence of comorbidities, and the natural history of COPD in women and in non-smokers.
Despite the fact that COPD is generally diagnosed during the sixth decade of life, all studies agree that onset is earlier,9 but it is not diagnosed until symptoms are perceived by the patient at more advanced ages. In this respect, a recent study has shown that the path to COPD differs, and that a significant proportion of cases that are detected in adulthood already have reduced lung function at younger ages.10 However, little is known about COPD in its early stages. Population studies, such as the European Community Respiratory Health Survey11 and EPISCAN in Spain,12 estimated a prevalence of 3.6% and 3.8% (4.4% in men and 3.2% in women), respectively, in individuals between 40 and 49 years of age. However, the factors determining the development of COPD from its initial stages are unknown. Certain studies have found that the decline in lung function is much more accelerated in the early stages than in the advanced stages of the disease,13 especially among symptomatic patients.14 Also of interest is the association of the initial COPD with other illnesses, such as depression,15 cardiovascular problems, and an increased prevalence of lung cancer.16,17
The lack of validated biomarkers for predicting and monitoring response to treatment among patients has been one of the major limitations in determining its natural history. FEV1, the universally accepted parameter for diagnosing and staging COPD, correlates poorly with symptoms in the early stages of the disease.18 Imaging markers, such as computerized tomography, would also be needed for the longitudinal assessment of structural changes in COPD.18 Club cell secretory protein-1619 and the surfactant protein D20 have been proposed as biological biomarkers of the disease.21–23 Other inflammatory markers, such as C-reactive protein, have also been associated with the systemic effects and prognosis of COPD.24 However, as yet, none has been validated as a marker of progression in patients with early-stage disease. Other types of progression markers, such as microbiological activity, persistent environmental exposure, and genetic factors, have not yet been explored in depth.
It is, then, of utmost importance to study patients with COPD from the early stages to determine the natural history of the disease and to design interventions aimed at modifying its prognosis.
Thus, the main objectives of the EARLY COPD study are the following:
- 1.
To characterize a COPD population of young adults from a multidimensional point of view;
- 2.
To compare these patients with smokers with normal lung function; and
- 3.
To create a cohort of young COPD patients who will be followed in the long term to improve understanding of the natural history of the disease, and to evaluate the parameters associated with the development of the disease during follow-up of the risk population, i.e., smokers in the control group, to determine which variables are associated with greater progression and worse prognosis.
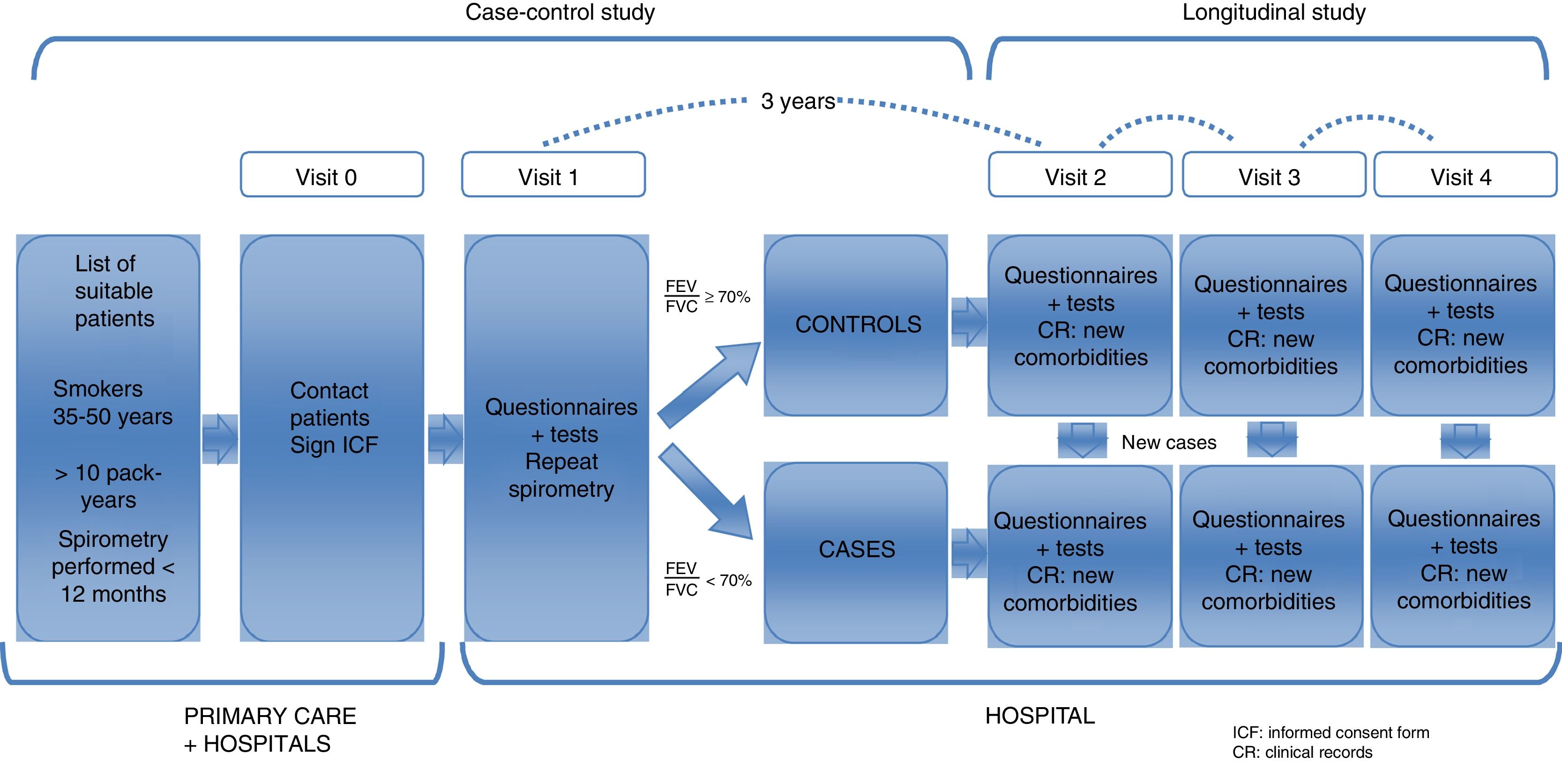
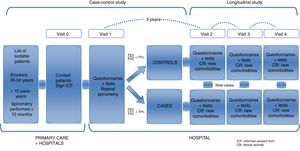
This is a 2-phase multicenter study. A case–control study was conducted to characterize a young population of COPD patients and to compare them with smokers with normal lung function (objectives 1 and 2). These same subjects will constitute a well-characterized cohort of young subjects, smokers or former smokers with and without COPD, that will be subsequently followed in a second study phase. This will allow for a longitudinal study of disease progression (objective 3) (Fig. 1).
Study PopulationSubjects were selected from the lists of approximately 30 primary healthcare centers and 12 hospitals in 8 Spanish autonomous communities. The visits were conducted in hospitals. The sites and principal investigators in each area are listed in Annex 1.
Selection of ParticipantsBetween March 2015 and March 2017 (24 months), a random sample of 311 subjects was recruited at a rate of 1:2 (101 cases and 210 controls). The inclusion criteria were smokers or former smokers (>10 pack-years), age between 35 and 50 years of age, and ability to perform forced spirometry. The cases were individuals with obstructive respiratory impairment, FEV1/FVC<70%. The controls had a normal spirometry, FEV1/FVC≥70%. Both cases and controls were selected at random among young smokers appearing in the lists of primary care or external consultations of the participating hospitals. If a previous spirometry was not available, it was always performed in visit 1, in the hospital where the subject was classified as a case or control, depending on the results of the spirometry.
The exclusion criteria for the cases were the following: active cancer, conditions that might interfere with possible future follow-up (frequent changes of residence, psychiatric problems), chronic inflammatory diseases or autoimmune diseases under treatment, cystic or saccular bronchiectasis, tuberculosis or other active pulmonary infection, and interstitial lung disease.
The exclusion criteria for controls were the same as for the cases, with the additional exclusion of a previous diagnosis of asthma and/or alpha-1-antitrypsin deficiency.
Individuals with symptoms of exacerbation (fever, cough, increased sputum volume and/or purulence, acute rhinitis) in the 8 weeks prior to the study visit were excluded from both groups.
The project was approved by the ethics committee of all participating centers. All subjects received written information about the objectives of the study and signed both the study consent form and a separate consent form for genetic analyses and the follow-up study. Participants’ data were anonymized and processed in accordance with the Data Protection Act, according to Regulation (EU) No 2016/679 of the European Parliament.
Study OrganizationA scientific committee consisting of pulmonologists, primary care physicians and epidemiologists was formed for study preparation and consultation. A strategic alliance was also established between the Center for Biomedical Research Network in Respiratory Diseases (CIBERES), the Center for Research in Environmental Epidemiology (CREAL, currently the Institute for Global Health of Barcelona [ISGlobal]), the SEPAR COPD Integrated Research Program, and the Primary Care Respiratory Group (GRAP). A local coordinator was appointed for each referral hospital, who remained in contact with the local primary care staff.
Follow-UpThe follow-up visit will take place 3 years after the baseline visit, as of March 2018. In this visit, the questionnaires and tests administered in the first visit will be repeated, with the exception of computed tomography, and with the addition of bioelectrical impedance analysis and a digital electrocardiogram. Ideally, we hope to be able to follow this cohort for about 5 or 10 more years.
Variables and ProceduresThe following information was initially collected from the subjects’ clinical history:
- •
Chronic diseases diagnosed.
- •
Current drug treatment, confirmed with the subject.
- •
Use of healthcare resources during the preceding year: the number of hospital admissions, number of visits to the emergency room, and number of outpatient medical consultations.
Subjects were then given an appointment at their local referral hospital to complete the following questionnaires and tests (Table 1):
- •
General questionnaire, developed from a variety of validated questionnaires, previously used in this type of subject and environment, containing the following sections:
- -
Sociodemographic data: sex, place and date of birth, marital status, and employment status.
- -
Early life factors: possible factors in early life that may be associated with the development of COPD in adulthood, such as respiratory infections before 5 years of age, prematurity, birth weight, mother smoking during pregnancy.9,25–27
- -
Diagnosis of chronic diseases: subjects were asked about diseases or symptoms that might affect or limit their normal daily activity.
- -
Non-pharmacological treatment: use of oxygen therapy, non-invasive ventilation or administration of continuous positive airway pressure.
- -
Smoking habit: age of onset, duration, quantity, and pack-years.
- -
Diet: questionnaire on adherence to the Mediterranean diet, where a score of less than 9 indicates a low adherence, associated with an increased risk of heart disease and an inflammatory pattern, that could also be present in patients with COPD.28,29
- -
Physical activity questionnaire: using the validated IPAQ questionnaire (short version), which consists of 4 questions concerning the time spent doing physical activity in the last 7 days.30
- -
Dyspnea according to the modified Medical Research Council dyspnea scale.31,32
- -
Brief COPD-PS questionnaire validated for COPD screening.33,34
- -
COPD Assessment Test (CAT) questionnaire to assess the health status of the participants, in both cases and controls.35
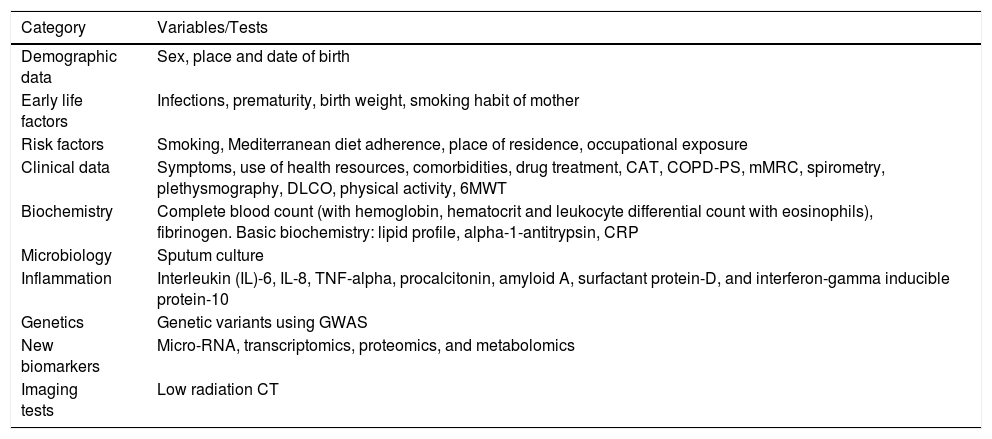
Study Variables/Tests.
| Category | Variables/Tests |
|---|---|
| Demographic data | Sex, place and date of birth |
| Early life factors | Infections, prematurity, birth weight, smoking habit of mother |
| Risk factors | Smoking, Mediterranean diet adherence, place of residence, occupational exposure |
| Clinical data | Symptoms, use of health resources, comorbidities, drug treatment, CAT, COPD-PS, mMRC, spirometry, plethysmography, DLCO, physical activity, 6MWT |
| Biochemistry | Complete blood count (with hemoglobin, hematocrit and leukocyte differential count with eosinophils), fibrinogen. Basic biochemistry: lipid profile, alpha-1-antitrypsin, CRP |
| Microbiology | Sputum culture |
| Inflammation | Interleukin (IL)-6, IL-8, TNF-alpha, procalcitonin, amyloid A, surfactant protein-D, and interferon-gamma inducible protein-10 |
| Genetics | Genetic variants using GWAS |
| New biomarkers | Micro-RNA, transcriptomics, proteomics, and metabolomics |
| Imaging tests | Low radiation CT |
6MWT: 6-minute walk test; CAT: COPD Assessment Test; COPD-PS: COPD Population Screening; CRP: C-reactive protein; DLCO: diffusion of carbon monoxide; mMRC: modified Medical Research Council scale.
In addition, all subjects who participated in the study (cases and controls) performed the following tests in the hospital:
- •
Physical examination: height, weight, blood pressure, heart rate, and waist and hip circumference.
- •
Six-minute walk test to measure exercise capacity. The same protocol, adapted from previously published guidelines, was strictly followed in all centers.36
- •
Complete lung function tests: forced spirometry and bronchodilator test, body plethysmography with lung volumes, determination of diffusing capacity for carbon monoxide and inspiratory and expiratory capacities.
- •
All procedures were standardized in accordance with the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) manual of procedures for assessing lung function.37,38 The bronchodilator test was performed with the administration of 400mg salbutamol using a spacer, in accordance with GOLD recommendations.39
- •
A low radiation computed tomography was performed to determine the presence of emphysema, bronchiectasis, coronary calcification, and pulmonary artery and aorta vascular diameters.
- •
Sputum culture. A sample of spontaneous sputum was obtained and divided into 2 parts: one was frozen at −80°C for subsequent microbiological and molecular techniques (including markers of inflammation and remodeling). The other part was processed with Murray staining and conventional culture, preferably quantitative, of potentially pathogenic micro-organisms in the microbiology laboratory.
- •
Blood sample for genetic and biological determinations: all participants provided a blood sample for a standard laboratory testing, conducted in each center (complete blood count, cholesterol, triglycerides, total proteins, albumin, prothrombin time, fibrinogen, C-reactive protein, erythrocyte sedimentation rate, and alpha-1-antitrypsin levels). Samples of serum, plasma, and formed elements were also obtained and immediately frozen and stored at −80°C for the analysis of biomarkers such as:
- -
Inflammatory markers in blood: interleukin (IL)-6, IL-8, TNF-alpha, procalcitonin, amyloid A, surfactant protein-D, and interferon-gamma inducible protein-10.
- -
Genetic markers: a genome-wide association study (GWAS) will be performed to evaluate the presence of genetic variants previously involved in disease susceptibility, and new genetic associations with different phenotypes/subtypes of the disease will be explored. DNA from cells obtained at the time of inclusion will be used.
- -
New biomarkers: micro-RNA, transcriptomics, proteomics, and metabolomics.
Data collection quality control was performed in the different participating centers both prior to the beginning of the study, when the staff responsible for data collection were trained, and before each phase. Quality control was also facilitated by the creation of an online database. The ranges for each of the variables are incorporated in the database design.
Sample SizeThe sample size was calculated using the GRANMO program 7.1040 taking several variables, such as FEV1 (percentage and liters), body mass index, or the distance walked in 6min as outcome measurements. The calculations were based on descriptive data obtained from studies with COPD patients from the same geographical area.41 We also took into account studies that collected information on FEV1 progress in various types of patients.42,43 Thus, for the longitudinal follow-up of the participants, taking an alpha risk of 0.05 and a beta risk of less than 0.10, in a 2-tailed comparison, and assuming a rate of loss to follow-up of 15%, a minimum of 99 subjects in each group would be required to identify a statistically significant difference between cases and controls of 300ml or more in the FEV1 variable.
Statistical Analysis PlanThe initial analysis will be a descriptive study of all variables included. The results of the continuous variables will be presented as mean, standard deviation, and the number of valid cases. For categorical variables, the number of cases in each category and frequency compared to the total number of answers will be used.
Moreover, the characteristics of the COPD patients will be described in detail and compared with the control group in a 2-tailed analysis. Finally, a logistic regression model will be constructed to determine which variables are associated with the development of COPD in young patients.
In all the statistical tests, statistical significance will be set at 0.05. Statistical analyses will be performed using Stata (www.stata.com).
DiscussionThe EARLY COPD project aims to generate a multidimensional characterization of a population of young patients with COPD, and to compare it with smokers with normal lung function. An additional objective is to establish a cohort of young patients with COPD that can be followed in the long term, to gain greater insight into the natural history of the disease. Both genetic and environmental elements are interrelated in the pathogenesis of COPD. A number of mechanisms that can interact in a patient are involved in the development of the disease, including poor lung development, the presence of infections in childhood, atopy, chronic inflammatory response of the airways, the role of recurrent infections during exacerbations, imbalance between proteolytic and anti-proteolytic activity leading to the destruction of tissue, and others. The EARLY COPD study aims to improve understanding the relative importance of these different pathogenic mechanisms in the development of the natural history of the disease. Moreover, by following a risk population (smoker controls), the study aims to determine which variables are associated with the subsequent development of COPD. Finally, we hope to identify different biomarkers as potential indicators of disease progression in the early phases, which will also be of vital importance in improving early diagnosis. The results of biomarkers obtained in this study will be compared with those obtained using similar methodology in the BIOMEPOC study, which examined a relatively advanced stage of the disease.
One of the greatest strengths of this project is that it is the result of a strategic alliance between various expert centers in epidemiology and respiratory health that has brought together both hospital and primary care professionals. It is a project involving various health centers throughout Spain (using the same standardized protocol), so we can assume that the results are quite representative of the regions under study.
One possible limitation of the study is, first and foremost, the considerable difficulty we encountered in recruiting cases that met the inclusion and exclusion criteria. The need for young patients with COPD made recruitment very difficult, due to the low prevalence of this disease among this population. Another possible limitation may be associated with the setting in which our subjects were recruited, since we initially planned to recruit COPD patients from primary care (patients between 35 and 50 years attending health centers), but finally, due to the difficulties encountered in selecting individuals in primary care, we also began to recruit patients in hospitals. This may also have modified the type of patients studied, as the patients recruited in hospitals may have had more severe disease.
To sum up, the EARLY COPD study will improve knowledge of the natural history of COPD and its determinants in the early stages of the disease, enabling the design of interventions aimed at modifying disease prognosis. Furthermore, the more specific objective of identifying different biomarkers as potential indicators of disease progression in early COPD will also be of great importance in improving the diagnosis and treatment of this disease.
FundingThis study was funded in part by a collaboration agreement between Boehringer-Ingelheim and CIBERES. It is included in the SEPAR COPD Integrated Research Program.
Conflict of InterestsAlicia Borras Santos has received honoraria in the last three years for speaking engagements, scientific consultancy, participation in clinical trials, and drafting of publications from (in alphabetical order): Boehringer Ingelheim, Esteve and Novartis. Borja G. Cosio has received honoraria in the last three years for speaking engagements, scientific consultancy, participation in clinical trials, and drafting of publications from (in alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Menarini, Novartis, Rovi, Teva and Zambón.
José Luis López-Campos has received honoraria for speaking engagements, scientific consultancy, participation in clinical trials, and drafting of publications from (in alphabetical order): Almirall, AstraZeneca, Bayer, Boehringer Ingelheim, Cantabria Pharma, Chiesi, Esteve, Faes, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, MSD, Novartis, Pfizer, Rovi, Teva, and Takeda. Miguel Román Rodríguez has received honoraria in the last three years for speaking engagements, scientific consultancy and participation in clinical trials from (in alphabetical order): AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, Rovi, and Teva. Sergi Pascual Guárdia has received honoraria in the last three years for speaking engagements, and participation in clinical trials from (in alphabetical order): Almirall, AstraZeneca, Boehringer Ingelheim, Esteve, Ferrer, GlaxoSmithKline, Menarini, Novartis, and Teva. Joaquim Gea has received honoraria in the last three years for speaking engagements and scientific consultancy from (in alphabetical order): AstraZeneca and Boehringer Ingelheim. He has also received unrestricted grants for research or teaching from ALK, Astra-Zeneca, Boehringer Ingelheim, Chiesi, Menarini, Mundipharma, and Novartis. Ciro Casanova has received honoraria in the last three years for speaking engagements, scientific consultancy, participation in clinical trials, and drafting of publications from (in alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Gebro, GlaxoSmithKline, Menarini, Novartis, Rovi, and Teva. Juan José Soler-Cataluña has received honoraria for speaking engagements, scientific consultancy, and participation in clinical studies from Air Liquide, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GSK, Menarini, Mundipharma, Novartis, Rovi, Sandoz, Teva, and Zambon. G. Peces-Barba has received honoraria in the last three years for speaking engagements, scientific consultancy, participation in clinical trials, and drafting of publications from (in alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, GlaxoSmithKline, and Menarini. Salud Santos has received honoraria in the last three years for speaking engagements, scientific consultancy, participation in clinical trials, and drafting of publications from (in alphabetical order): AstraZeneca, Boehringer Ingelheim, Laboratorios Ferrer, GlaxoSmithKline, Gebro Pharma, Menarini, Novartis, Pfizer, and Rovi.Pedro J. Marcos has received honoraria in the last three years for speaking engagements, scientific consultancy and participation in clinical trials from (in alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Gebro, GlaxoSmithKline, Menarini, MSD, Mundipharma, Novartis, Rovi, Roche, Sandoz, and Teva. Rosa Faner has received honoraria in the last three years for speaking engagements and scientific consultancy from (in alphabetical order): Chiesi, GlaxoSmithKline, and MSD. Alvar Agusti has received honoraria in the last three years for speaking engagements, scientific consultancy and participation in clinical trials from (in alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Menarini, Novartis, Nuvaira, and Teva. The other authors state that they have no conflict of interests.
EARLY COPD Study Research Group:
- -
Hospital Son Espases (Mallorca): Borja Garcia-Cosio Piqueras, Rocío Cordova Diaz, María Magdalena Pan Naranjo, Joan Palmer Sancho y Miguel Román Rodríguez.
- -
Hospital Clínic (Barcelona): Alvar Agustí, Rosa Faner Canet, Joan Albert Barberà, Josep Roca Torrent, Yolanda Torralba Garcia, Jorge Moises Lafuente, Anna Maria Pedro Pijoan, Amparo Hervas Docón, Carmen Herranz y Núria Sanchez Ruano.
- -
Hospital del Mar (Barcelona): Joaquim Gea Guiral, Diego A. Rodríguez Chiaradía, Anna Rodó-Pin, Clara Martín-Ontiyuelo, Mireia Admetlló, Concepción Ballano Castro, Laura Gutiérrez Martín, José Ignacio Aoiz Linares, Sergi Pascual-Guardia y Marta Mourelo Cereijo.
- -
Fundación Jiménez Díaz (Madrid): Germán Peces-Barba Romero, José Fernández Arias, Carolina Gotera Rivera, Manuel Martin Bernal, Guillermo Gallardo Madueño, Andrés Alcázar Peral, Carmelo Palacios Miras, María Teresa Pinedo Moraleda, María Belén Torres Labandeira, Mercedes Colomo Rodríguez, María Concepción Rodríguez Gallego, Carmen Lobon Agundez, Mónica Nácher Conches, María José Mansilla y Rosario Serrano Martín.
- -
Hospital 12 Octubre (Madrid): Carlos J. Álvarez Martínez, Marta Padilla Bernáldez y Jesús Molina París.
- -
Hospital Parc Taulí (Sabadell): Laura Vigil Giménez, Eduard Monsó Molas, Laia Seto Gort, Montserrat Baré Mañas y Anna María Fabra Noguera.
- -
Hospital Virgen del Rocío (Sevilla): José Luis López Campos, Carmen Calero Acuña y Laura Carrasco Hernández.
- -
Hospital Universitario de Bellvitge (Hospitalet de Llobregat, Barcelona): Salud Santos Perez, Montserrat Navarro, Elisabeth Serra, Ferran Ferrer Keysers, Damaris Batallé, M. Dolores Peleato Catalan, Albert Dorca y Javier Burgos, Marina Bosch Ventura y José Carlos Ruibal
- -
Hospital Arnau de Vilanova (Valencia): Juan José Soler-Cataluña, Noelia González García y Lourdes Sánchez Sánchez
- -
Hospital Universitario Central de Asturias (Oviedo): Cristina Martínez González, Amador Prieto Fernández y Susana Martínez González
- -
Hospital Candelaria (Canarias): Ciro Casanova Macario y Delia Mayato
- -
Hospital Universitario de A Coruña: Pedro J. Marcos Rodríguez, Luis Domínguez Juncal, Rosario Timiraos Carrasco y Rosa García Palenzuela.
The full list of the authors of the Research Group of the EARLY COPD study is shown in Annex 1.
Please cite this article as: Borràs-Santos A, Garcia-Aymerich J, Soler-Cataluña JJ, Vigil Giménez L, Gea Guiral J, Rodríguez Chiaradía D, et al. Early COPD: determinantes de la aparición y progresión de la enfermedad pulmonar obstructiva crónica en adultos jóvenes. Protocolo de un estudio caso-control con seguimiento. Arch Bronconeumol. 2019;55:312–318.