Recent research on the relationship between the immune system and cancer has revealed the molecular mechanisms by which cancer cells co-opt certain T cell receptors which block the cytotoxic response to defend themselves from the antitumor immune attack. These findings have helped identify specific targets (T cell receptors or their corresponding ligands) for the design of monoclonal antibodies that can unlock the immune response.
These drugs, known as immune checkpoint inhibitors, have shown efficacy in metastatic melanoma and kidney cancer, and have been successfully tested in non-small cell lung cancer in recent trials. Immune checkpoint inhibitors were included in clinical practice as a second-line option after an initial chemotherapy (CT) regimen, and in the last year positive results have been reported from randomized trials in which they were compared in first line with standard CT. Responses have been surprising and durable, but less than 20%–25% in unselected patients, so it is essential that factors predicting efficacy be identified. One such biomarker is PD-L1, but the different methods used to detect it have produced mixed results.
This non-systematic review discusses the results of the latest trials, the possibilities of incorporating these drugs in first-line regimens, the criteria for patient selection, adverse effects, and the prospects of combinations with conventional treatment modalities, such as CT, radiation therapy, and antiangiogenic agents.
Investigaciones recientes sobre la relación entre el sistema inmune y el cáncer han desvelado los mecanismos moleculares mediante los cuales las células neoplásicas aprovechan algunos receptores de los linfocitos T, con función inhibitoria de la respuesta citotóxica, para defenderse del ataque inmune desarrollado frente a ellas. Estos hallazgos han permitido identificar dianas precisas (receptores de los linfocitos T o ligandos que se acoplan a ellos) frente a los que se han diseñado anticuerpos monoclonales, capaces de desbloquear la respuesta inmunitaria.
Estos fármacos (immune check point inhibitors), de eficacia demostrada en el melanoma metastásico o el carcinoma renal, han sido probados con éxito frente al carcinoma de pulmón no microcítico en ensayos recientes. Tras su aprobación e incorporación a la práctica clínica en 2.ª línea después de una pauta inicial de quimioterapia (QT), se han comunicado en el último año resultados positivos en ensayos aleatorizados que los comparaban con QT estándar en 1.ª línea. Se han observado respuestas sorprendentes y duraderas, aunque no superan el 20-25% en pacientes no seleccionados, por lo que es crucial detectar rasgos predictivos de eficacia, como el biomarcador PD-L1, si bien los diferentes métodos para su detección han producido resultados dispares.
En esta revisión no sistemática se discuten los resultados de los últimos ensayos, las posibilidades de incorporar estos fármacos en primera línea, los criterios de selección de pacientes, los efectos adversos y las perspectivas de su empleo asociados a modalidades terapéuticas tradicionales como QT, radioterapia o antiangiogénicos.
The idea of stimulating and enhancing the immune response to cancer was first conceived by Paul Ehrlich more than a century ago,1 and has aroused the interest of many authors, but only in the last few years has the notion taken shape in the form of therapeutic advances that can be used in the clinic.2 For years, the concept map for this line of research was based on introducing a series of generally non-specific antigens that would induce an immune response in the patient that could arrest the growth of neoplastic cells. The aim was to emulate the success of traditional vaccines in the prevention of allergies and infectious diseases. Multiple vaccines derived from inactivated neoplastic cells were tested, and there was even some experimentation involving the introduction of infectious agents into tumors in the hope of activating the immune system.3,4
Although lung cancer (LC) has long been considered non-immunogenic, some findings suggest otherwise. For example, the incidence of LC and other cancers is very high in organ transplant recipients with induced immunosuppression and patients with human immunodeficiency virus (HIV) infection.5 The presence of tumor-infiltrating lymphocytes in resected LC specimens has also been associated with better prognosis. Moreover, many non-small cell LCs (NSCLC) are known to produce antigens that can induce an immune response.2,5,6
Anticancer vaccines and immune stimulating substances (interferon and various interleukins) that show promising preliminary results against various tumors, including LC,7 have been developed, but their widespread use, except in metastatic melanoma and renal cell carcinoma, has been limited by lack of specificity and adverse effects.3
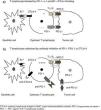
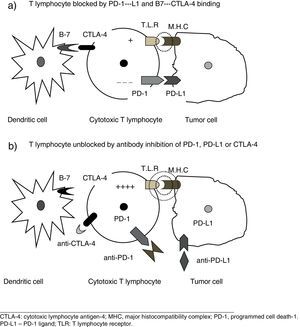
New Pathogenic Concepts of Immunity and CancerRecent research6–8 has led to a deeper understanding of the complex relationships between the immune system and tumor cells, and of the molecular mechanisms by which tumor cells can avoid destruction by T cells. It has been shown that other stimulatory signals, in addition to the presentation of antigen by dendritic cells, are required to activate cytotoxic T cells, and pathways that block those signals and thus promote tumor growth have been identified. The theory of immune surveillance, referring to the mere recognition by the body of cancer cells as foreign, has been replaced by the concept of immune editing, which hypothesizes that the immune system interacts with the tumor in 3 phases: (1) recently developed cancer cells are eliminated by innate and adaptive immune mechanisms; (2) some cells that can avoid this immune attack enter into a dormant state; and (3) finally, these cells can escape immune control and proliferate, leading to clinically apparent disease, a feature now considered a hallmark of cancer cells.6 In other words, the tumor evades attack by the T cells, taking advantage of certain immune checkpoints. Therapeutic targets of special interest include the programmed cell death-1 (PD-1) receptor, its associated ligands (PD-L1), and cytotoxic T-lymphocyte antigen-4 (CTL4-A). Effective therapeutic agents aimed at these targets are already available.6–8
In addition to these regulatory pathways, the immune profile of an individual depends on multiple factors, including the intrinsic properties of the tumor (genetic makeup, cytokine secretion, etc.) and extrinsic circumstances, such as gut microbiome, presence of infections, and exposure to environmental carcinogens. The bottom line of all these influencing factors is a specific anti-cancer immunity status that determines an individual's potential response to immunotherapy agents.8
New Therapeutic Concepts. Immune Checkpoint InhibitorsCancer cells can be prevented from using these inhibitory pathways by blocking the above-mentioned receptors or the corresponding ligands with specific monoclonal antibodies.2–8 These agents, by their mechanism of action, neutralize the checkpoints used by the tumor cells, and unblock the ability of the lymphocytes to destroy them (Fig. 1), so they could possibly be called immune checkpoint inhibitors, a neologism that we will provisionally use in this paper.
Preliminary positive results with immune checkpoint inhibitors in randomized trials were first obtained in metastatic melanoma with ipilimumab, a monoclonal antibody that neutralizes the CTLA-4 receptor which inhibits T cells. A trial published in 2010 showed that ipilimumab in second-line treatment was superior to the comparative regimen based on the gp100 vaccine (median survival 10.1 and 6.4 months, respectively).9 These outcomes helped this drug receive rapid approval by the regulatory authorities in the U.S., and since then, numerous studies have been performed in other cancers. Table 1 shows some of these drugs and their targets.
Preliminary Trials With Immune Checkpoint Inhibitors in Non-Small Cell Lung CancerThe first indications that these agents were effective in LC were obtained in 2 phase I clinical trials that included NSCLC and other tumors. Different doses of the study drugs were tested (anti-PD-1 in 1 trial and anti-PD-L1 in the other). Favorable outcomes were observed in 18% of NSCLCs, and in up to 36% of PD-L1-positive tumors. Most of these responses lasted more than 1 year, with an acceptable safety profile and a 14% incidence of WHO grade 3/4 adverse events.10,11 These results were quickly followed by initial randomized trials in the second-line treatment of NSCLC patients who had failed or relapsed after a first line of standard chemotherapy (CT) based on platinum doublets; one of the trials included squamous tumors only, and the other non-squamous tumors only. Response rates and overall survival were significantly better with the study drug (nivolumab) than with the control docetaxel-based CT. Moreover, significantly fewer severe side effects were reported in the group treated with nivolumab (Table 2).12,13 The lack of benefit in progression-free survival (PFS) can be explained by the peculiar effects of these drugs, which sometimes initially produce an apparent increase in tumor volume. This issue is discussed below in the section on evaluation criteria for response. Subsequent trials have confirmed the effectiveness of other immune checkpoint inhibitors in the second-line treatment of NSCLC patients14,15 (Table 2), and the National Comprehensive Cancer Network (NCCN) (version 6.2017) recommends their use in this setting, citing grade 1 evidence.16 The most recent drugs (durvalumab and avelumab) have shown promising results in phase I-II clinical trials17–19 (Table 1).
Immune Checkpoint Inhibitors (ICI) in Advanced NSCLC Randomized Trials After Relapse.
| No. | ICI vs CT | Strain | Response Rate (%) | Progression-Free Survival (Months) | Overall Survival (Months) | Severe Adverse Effectsa (%) | |
|---|---|---|---|---|---|---|---|
| CheckMate 017 | 272 | Nivolumab vs docetaxel | Squamous | 20% nivol. vs 9% docet. | 3.5 nivol. vs 2.8 docet. | 9.2 nivol. vs 6.0 docet. | 7% nivol. vs 55% docet. |
| CheckMate 057 | 582 | Nivolumab vs docetaxel | Non-squamous | 19% nivol. vs 12% docet. | 2.3 nivol. vs 4.2 docet. | 12.2 nivol. vs 9.4 docet. | 10% nivol. vs 54% docet. |
| Keynote-010 | 850 | Pembrolizumabb vs docetaxel | Squamous and non-squamous | 3.9 & 4.0 nivol. vs 4.0 docet. | 10.4 & 12.7 vs 8.5 | 13% & 16% vs 35% | |
| OAK | 1034 | Atezolizumab vs docetaxel | Squamous and non-squamous | 18% atezo. vs 16% docet. | 2.8 atezo. vs 4.0 docet. | 13.8 atezo. vs 9.6 docet. | 15% atezo. vs 43% docet. |
The expectations raised by these drugs has prompted intense research activity, and relevant information is already available on their efficacy in first line: in a phase I trial, nivolumab (at doses of 3mg/kg body weight every 2 weeks), used as initial monotherapy in 52 patients with NSCLC, yielded objective responses in 23% of the series, and in 28% of the subgroup with PD-L1 biomarker expression. Median overall survival was 19.4 months, and the severe adverse effect rate was 19%.20
Numerous first-line phase III trials in NSCLC are currently ongoing,21 and the results of some completed trials have already been published (Keynote-024, Table 4). One of these studies compared the anti-PD-1 immune checkpoint inhibitor pembrolizumab with platinum-based CT, although one of the inclusion criteria for the study was PD-L1 expression in over 50% of tumor cells, a criterion met by 20%–25% of patients with advanced NSCLC. This trial (Keynote-024, Table 2) showed pembrolizumab to be superior in terms of overall survival and PFS, and less toxic than CT.22 On the basis of these results, the NCCN (version 3.2017) recommends using this immune checkpoint inhibitor when starting first-line treatment of NSCLC in patients whose tumor expresses the high concentrations of PD-L1 required in the above-mentioned study.16 In the other randomized study (presented at a congress, but not yet published) (CheckMate 026), nivolumab was compared to standard CT; PD-L1 expression was required in at least 1% of tumor cells. Overall survival was similar in both groups, while the immune checkpoint inhibitor showed less toxicity.23 Although further analysis is required, the failure of nivolumab to show superiority in this trial may be due to the excessively low threshold for PD-L1 expression (1%) required for inclusion in the original design.
Biomarkers Predicting Favorable ResponseImmune checkpoint inhibitors sometimes produce remarkable results, including long-term complete remissions, but objective responses are observed in at most 15%–20% of unselected patients.4,24 It is of the utmost importance, therefore, to identify some clinical or tumor marker to predict which patients are more likely to benefit. Since some of these drugs are monoclonal antibodies designed to inhibit the PD-1 receptor, as mentioned above, it seems reasonable to expect that the concentration of PD-L1 (ligands that bind to this receptor) expressed in tumor or immune cells would be useful for predicting efficacy. However, the wide range of reagents developed by the different companies and the diverse cut-off points established by different authors have generated conflicting results.25,26 In the CheckMate 017 trial (Table 2), conducted in patients with squamous cell carcinoma, PD-L1 concentrations did not affect outcomes12; in contrast, in CheckMate 057, patients with non-squamous cell carcinoma and PD-L1 concentrations higher than 10% in tumor biopsies had longer mean overall survival than those with levels lower than 10% (19.4 and 9.9 months, respectively).13 The overall assessment of the findings from these and other studies suggests that this biomarker has a predictive value for response, and cooperative efforts are being made to harmonize, standardize and validate analytical techniques that would simplify the use of this biomarker in clinical practice.25
In addition to PD-L1, other factors may have predictive value. For example, it seems that the greater the number of mutations in a tumor, the more sensitive it is to these drugs, because the number of antigens that can be recognized by the immune system will also be greater. For this reason, one proposal is to sequence the complete tumor genome or a preselected panel of representative genes to evaluate the mutational load.25,26 This hypothesis is supported by the fact that cancers attributed to excessive exposure to ultraviolet light (skin cancer) or tobacco smoke (lung cancer), which have a large number of mutations, respond best to these drugs. In NSCLC trials, when responses were analyzed according to different characteristics, smokers were found to respond better than non-smokers.20,22,25 Although there are still no clear recommendations in this respect, in the absence of available biomarkers, this epidemiological finding may serve to guide difficult therapeutic decisions in clinical practice.
Criteria for the Evaluation of ResponsePFS is an essential criterion for assessing the efficacy of a therapeutic regimen in patients with advanced NSCLC. However, these drugs have shown significant discrepancies between PFS and overall survival which can be explained by the specific effects of unblocking the immune reaction. Indeed, an apparent increase in tumor volume can be observed, due to lymphocytes infiltrating the tumor, reflecting the efficacy of the immune checkpoint inhibitory effect of the drug. This paradoxical phenomenon which mimics disease progression, but which in fact is the opposite, is called “pseudoprogression”,2 and only after a long period of close monitoring of the patient will the reduction in tumor size become apparent. For this reason, it has been suggested that overall survival, and not PFS, is the criterion that most accurately reflects the real benefits.27,28 This peculiar tumor response also calls for a change in the conventional therapeutic evaluation criteria for solid tumors (RECIST criteria), which now should take into account the possibility of “pseudoprogression”.28,29 It may even be necessary to include different statistical parameters in the evaluation of PFS, since this phenomenon leads to crossing of the Kaplan–Meier curves that violates the proportional hazards assumption demanded by some tests.27
Combined Immune Checkpoint Inhibitor and Chemotherapy RegimensSince the antitumor mechanisms of action of immune checkpoint inhibitors and conventional CT differ radically, the idea of testing the combination of both drugs is attractive. This approach has been explored in 2 recent phase I and II trials: (a) CheckMate 012, in which platinum-based CT was combined with different doses of nivolumab, followed by maintenance with nivolumab until disease progression or unacceptable toxicity. Although only 52 patients were included, objective responses (between 33% and 47%) and overall survival at 2 years (between 25% and 62%) are encouraging30; and (b) Keynote-021, comparing pembrolizumab plus CT (carboplatin and pemetrexed) with the same CT regimen alone; both arms subsequently received maintenance pemetrexed. Favorable response rates were 55% and 19%, respectively, and adverse effects rates were similar.31
Immune Checkpoint Inhibitors in Small Cell Lung CancerSmall cell lung cancer is a very aggressive CT-sensitive tumor, closely related to smoking, with the consequent high mutational load. As such, it is in theory an excellent candidate for these new agents.32 Phase II studies combining ipilimumab (anti-CTL4-A) with platinum derivatives and etoposide (the usual regimen in this tumor) showed modest improvements in PFS, but in a large randomized trial the addition of ipilimumab to the standard CT regimen showed no benefit compared to CT alone.33 Recently, a combination of 2 immune checkpoint inhibitors (anti-CTL4-A and anti-PD-1) that act on different targets has produced objective responses in the range of 10%–19%, with acceptable toxicity, in patients with recurrent small cell lung cancer.34 In view of these results, the NCCN (Version 3.2017) recommends the use of these agents in the event of relapse within 6 months after the first treatment (level of evidence 2A).35
Contraindications. Immune-Related Adverse EffectsIn clinical trials, significantly fewer severe adverse effects (WHO grades 3 and 4) were reported with immune checkpoint inhibitors than with standard CT (Tables 2 and 3).12–15,22 However, the particular mechanism of action of these agents gives them a unique toxicity profile. The PD-1/PD-L1 pathway plays a crucial role in immune homeostasis and the suppression of T cell activity against autoantigens. Thus, if this pathway is blocked, an immune attack can be triggered, not only against the tumor but also against normal tissues, causing autoimmune disease.32,36,37 In most trials, a clinical history of autoimmune diseases, chronic HIV or hepatitis B or C infection, active interstitial lung disease or other diseases treated with corticosteroids was considered an exclusion criterion. However, although experience is limited, reports of isolated cases and small series in uncontrolled studies suggest that these drugs can even be used in these circumstances if patients are closely monitored, and that toxicities are manageable.36,37
Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Inhibitors Adverse Effects in Randomized Trials in LC Patients.
| Intensity of Any Grade % | Intensity WHO Grades 3–5% | |
|---|---|---|
| Common toxicity | ||
| Any effect | 63–73 | 16–26 |
| Hyporexia | 9–14 | 0–1 |
| Stomatitis | 2–4 | 0 |
| Nausea | 9–11 | 0–1 |
| Vomiting | 2 | 0.6 |
| Diarrhea | 7–14 | 1–4 |
| Anemia | 5 | 2 |
| Neutropenia | 0.6 | 0 |
| Rash | 4–13 | <1 |
| Pyrexia | 5–10 | <1 |
| Due to autoimmune mechanism | ||
| Any | 29.2 | 9.7 |
| Hypothyroidism | 4.0–9.1 | 0.0 |
| Thyroiditis | 2.6 | 0.0 |
| Hyperthyroidism | 4.0 | <1.0 |
| Pneumonitis | 4.0–5.8 | 2.6 |
| Colitis | 1.0–2.0 | 0–1.0 |
| Hypophysitis | 0.0–0.6 | 0–0.6 |
Randomized First-Line Trials With Immune Checkpoint Inhibitors (ICI) in Advanced NSCLC Patients Positive for PD-L1.
| No. | ICI vs CTa | Strain | Response Rate (%) | Progression-Free Survival (Months) | Overall Survival at 6 Months | Severe Adverse Effectsb (%) | |
|---|---|---|---|---|---|---|---|
| Keynote-024 | 825 | Pembrolizumab vs CTa | Squamous and non-squamous | 44.8% pembro. vs 27.8% CTa | 10.3 pembro. vs 6.0 CTa | 80.2 pembro. vs 72.4 CTa | 26.6% pembro. vs 53.3% CTa |
| CheckMate 026 | 541 | Nivolumab vs CTa | Squamous and non-squamous | No data available | 4.2 nivol. vs 5.9 CTa | 14.4 nivol. vs 13.2 CTa | 18% nivol. vs 51% CTa |
In the 2 first-line trials, only patients selected on the basis of biomarker PD-L1 expression were included.
The most common immune-related effects include skin rash, colitis, liver diseases, pneumonitis, and endocrine diseases, such as hypophysitis and thyroiditis (Table 3). If these conditions are suspected, infection or tumor progression should first be ruled out, and topical, oral or intravenous corticosteroids should be administered, depending on severity.36,37
Cost-Effectiveness AnalysisThe cost of immune checkpoint inhibitors varies depending on the country and the health system model, but they are generally very high: between $150,000 and $180,000 per quality-adjusted year of life.38,39 A recent economic analysis conducted in Switzerland concluded that at current prices, nivolumab is not cost-effective compared to docetaxel. Price reduction or a stricter selection of the population according to PD-L1 levels would be necessary.39
Prospects for the Future. Combinations With Chemotherapy and Radiation TherapyThe development of clinical applications of immune checkpoint inhibitors has gained momentum in the USA, because the Food and Drug Administration granted these drugs priority review status, thus speeding up the procedures for the evaluation and approval of new medications.25
Numerous studies are now exploring the use of these drugs in neoadjuvant and adjuvant treatment, and as consolidation therapy after an initial regimen of CT and radiation therapy. Preliminary data have also appeared which suggest that antiangiogenic agents (bevacizumab, nintedanib, etc.) can stimulate the immune system, and trials appear to indicate that the combination with immune checkpoint inhibitors produces synergy without substantially increasing toxicity.40 These combinations are still at an early stage of investigation, and the doses and optimal sequence of administration of each drug remain to be determined. However, research is now focusing primarily on the possible incorporation of these new drugs in first-line therapy and the selection of the right candidates in order to begin this paradigm shift in treatment. The most widely studied biomarker predicting a favorable response to date has been the immunohistochemical expression of PD-L1, although some trials have also shown positive results in patients with low or negative concentrations of PD-L1.28 The use of different antibodies and analytical methods has been an obstacle to the validation of this biomarker,25,26 prompting an international effort to standardize the procedures and to design a common, reliable method. The utility of other markers is also being investigated, and areas of interest range from smoking to the presence of tumor-infiltrating lymphocytes or determination of the mutational load by genomic sequencing of the tumor.26
Data available to date suggest that, in the medium term, it is very unlikely that these drugs will completely substitute platinum-based CT in the treatment of NSCLC, but the development of reliable markers may help us identify patients with a greater chance of benefitting from immune checkpoint inhibitors, whether in monotherapy, or in combination with other similar or different therapeutic modalities, including traditional CT.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Sánchez de Cos Escuín J. Nueva inmunoterapia y cáncer de pulmón. Arch Bronconeumol. 2017;53:682–687.