It is unclear whether low-risk patients with acute symptomatic pulmonary embolism (PE) should undergo echocardiogram.
MethodsWe performed a meta-analysis of studies that enrolled patients with acute low-risk PE to assess the prognostic value of echocardiographic diagnosis of right ventricular (RV) dysfunction for the primary outcome of short-term all-cause mortality, and the secondary outcome of short-term PE-related mortality. We used a random-effects model to pool study results, a Begg rank correlation method to evaluate for publication bias, and I2 testing to assess heterogeneity.
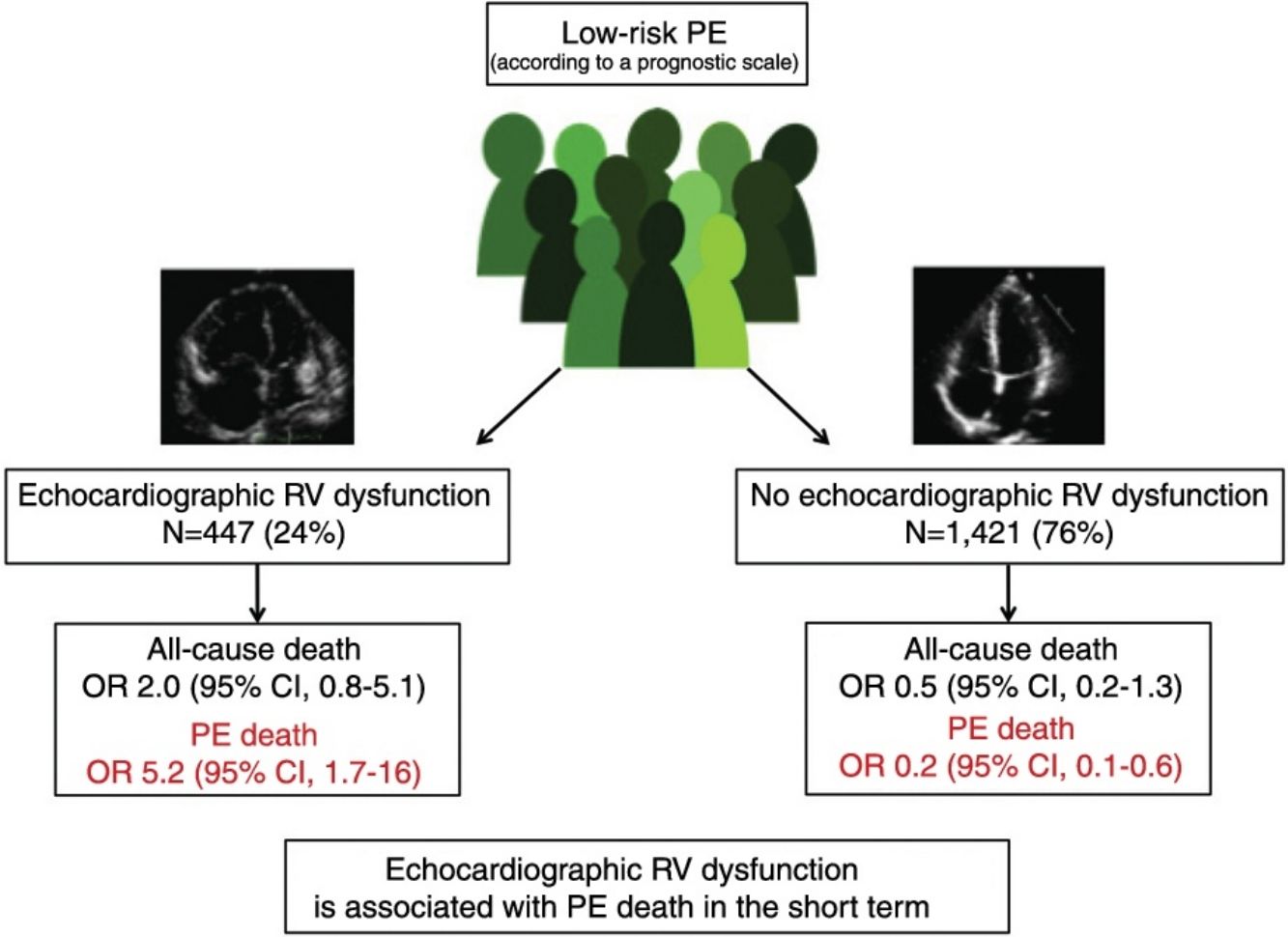
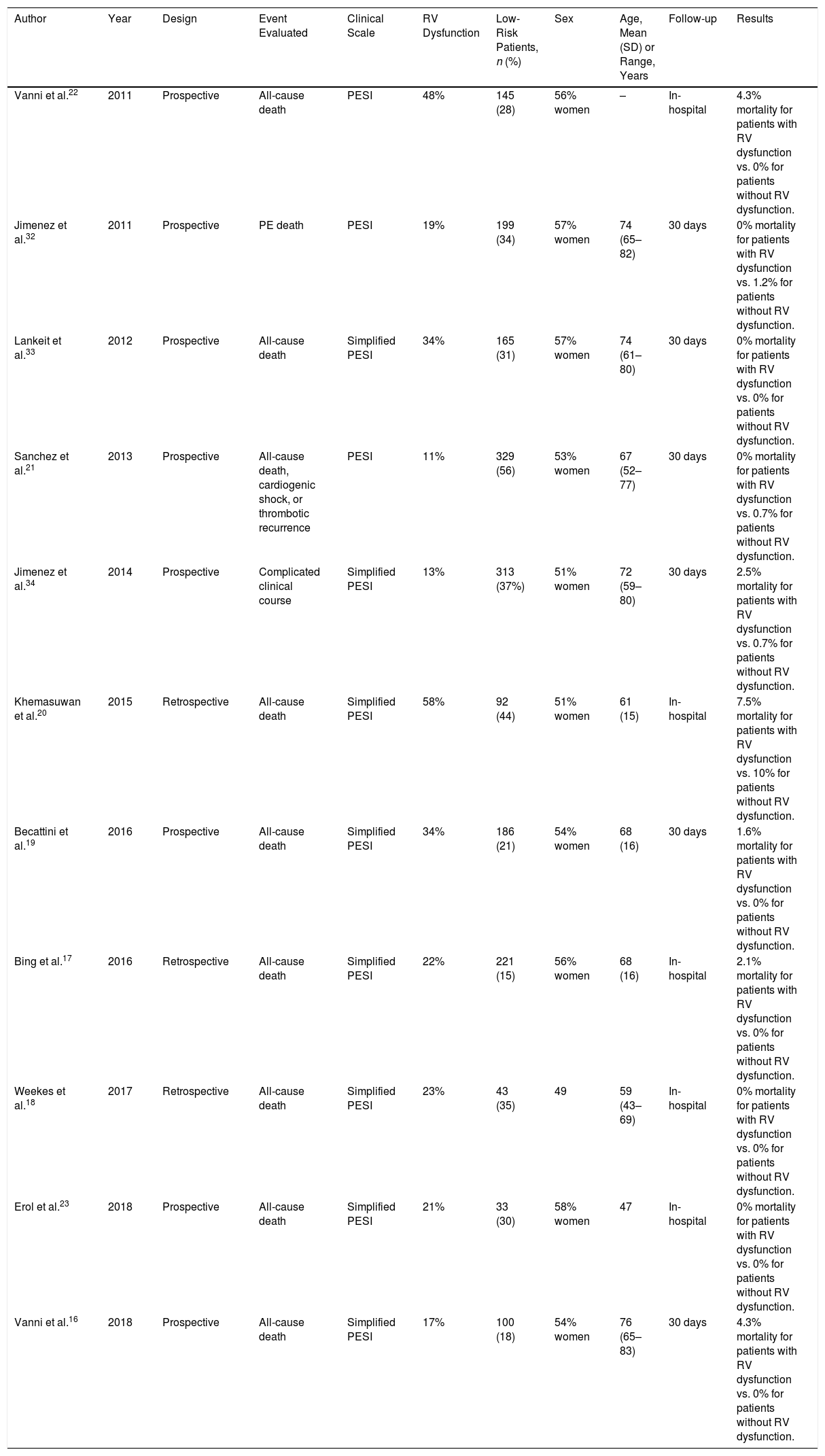
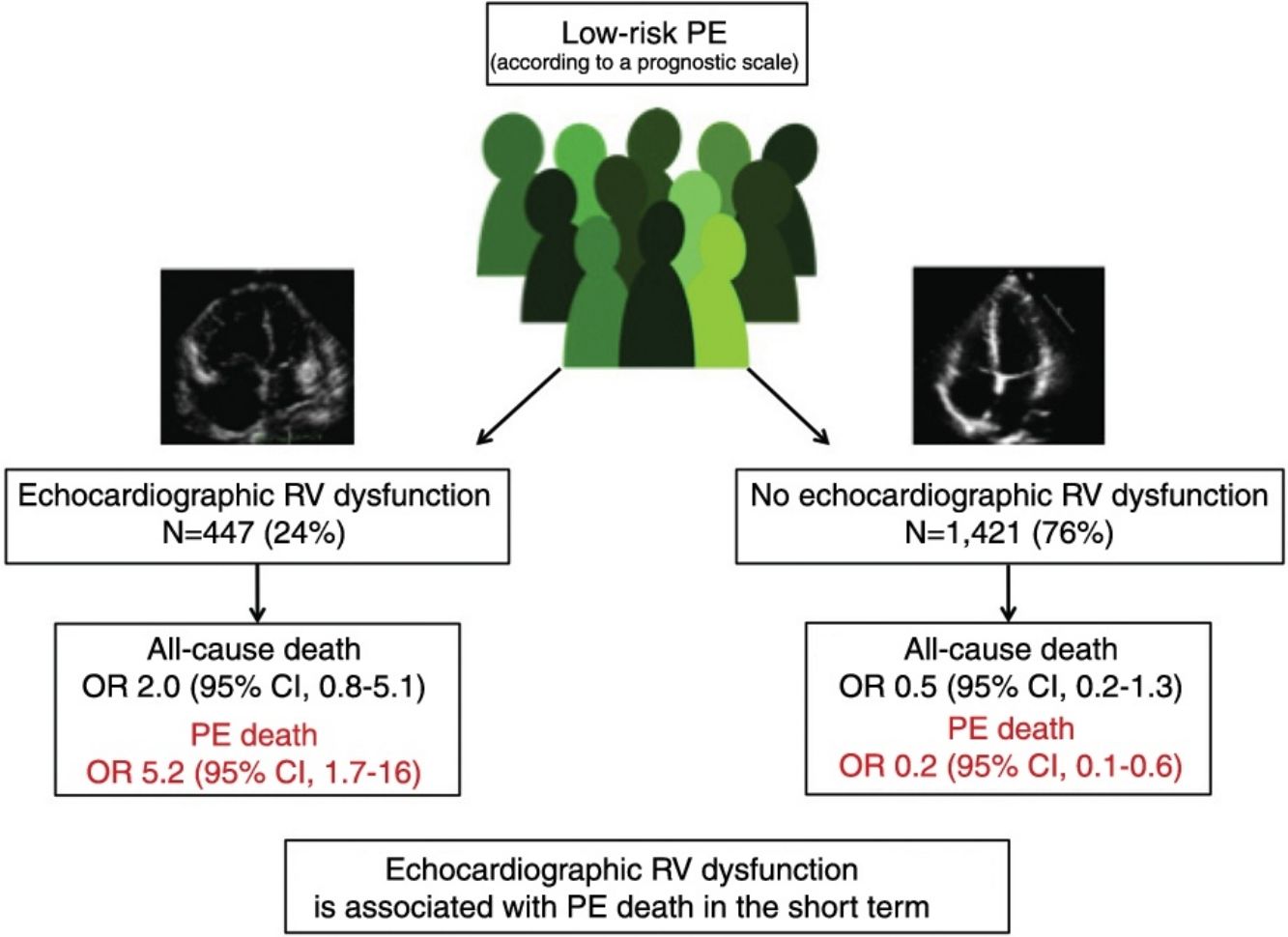
ResultsThe meta-analysis included a total of 11 studies with 1868 low-risk PE patients. Ten of the 447 (2.2%; 1.1%–4.1%) low-risk patients with echocardiographic RV dysfunction died soon after the diagnosis of PE compared with 10 of 1421 (0.7%; 0.3%–1.3%) patients without RV dysfunction. RV dysfunction was not significantly associated with short-term all-cause mortality (odds ratio 2.0; 95% confidence interval, 0.8–5.1, P=.14; I2=8%). RV dysfunction was significantly associated with short-term PE-related mortality (odds ratio 5.2; 95% confidence interval, 1.7–16, P<.01; I2=0%).
ConclusionsIn patients with low-risk PE, echocardiographic RV dysfunction is not associated with all-cause mortality, but identifies patients with an increased risk for short-term PE-related mortality.
No se ha aclarado completamente si se debería realizar una ecocardiografía a los pacientes con tromboembolia de pulmón (TEP) de riesgo bajo.
MétodosRealizamos un metanálisis de estudios observacionales que incluyeron pacientes con TEP de riesgo bajo para determinar el significado pronóstico de la disfunción ecocardiográfica del ventrículo derecho (VD). El evento primario considerado fue la muerte por cualquier causa a corto plazo. El evento secundario fue la muerte por la propia TEP a corto plazo. Utilizamos un modelo de efectos aleatorios para combinar los resultados, la prueba de correlación de rangos de Begg para estimar el sesgo de publicación y la prueba de la I2 para evaluar la heterogeneidad de los estudios incluidos.
ResultadosIdentificamos once estudios observacionales que incluyeron 1.868 pacientes con TEP de riesgo bajo. Diez de los 447 (2,2%) pacientes con TEP de riesgo bajo y disfunción del VD fallecieron, comparado con 10 de los 1.421 (0,7%) pacientes sin disfunción del VD. La presencia de disfunción ecocardiográfica del VD no se asoció con la mortalidad precoz por cualquier causa (odds ratio 2,0; intervalo de confianza del 95%, 0,8-5,1, P=0,14; I2=8%), pero se asoció de forma significativa con la muerte por la propia TEP (odds ratio 5,2; intervalo de confianza del 95% 1,7-16, P<0,01; I2=0%).
ConclusionesEn pacientes con TEP de riesgo bajo, la disfunción ecocardiográfica del VD no se asocia significativamente a la muerte por cualquier causa pero se asocia de forma significativa a la muerte por la propia TEP.
Pulmonary embolism (PE) is a disease with a wide spectrum of clinical manifestations, prognoses, and treatments.1 In recent years, various prognostic tools have been validated for patients with acute symptomatic PE, including clinical scales, imaging tests, and cardiac biomarkers.2
The prognostic stratification of patients with PE identifies patients with acute symptomatic PE and a low risk of complications in the short term who might benefit from a shorter hospital stay or even home treatment of their disease.3 Clinical practice guidelines recommend the use of validated prognostic scales (i.e., the Pulmonary Embolism Severity Index4 scale, the simplified PESI scale5 or the Hestia criteria6) for the identification of low-risk patients.7 However, some studies suggest that such scales used in isolation are not sufficiently sensitive to safely identify PE patients with a negligible risk of complications in the short term.8–10
A recent meta-analysis analyzed the prognostic significance of right ventricular (RV) dysfunction in patients with low-risk PE.11 However, this systematic review combined studies that had used chest computed tomography (CT) or echocardiography indiscriminately to assess RV size/function. As the concordance between CT angiography and echocardiography is poor,12,13 the authors were unable to determine whether a transthoracic echocardiography should be requested in patients with low-risk acute PE.
The aim of this meta-analysis was to assess the prognostic significance of RV dysfunction determined by echocardiography in low-risk PE patients according to a well-validated clinical scale.
MethodStudy RetrievalTwo investigators (IA and DJ) performed independent literature searches for eligible articles published between October 2010 and May 2019 by way of a systematic review of the PubMed and Web of Science databases. Discrepancies between the two investigators’ results were resolved with the help of a third researcher (EM). The searches were made using the following strategy: («pulmonary embolism»[MeSH Terms] OR «pulmonary embolism»[All Fields]) AND («PESI»[All Fields] OR «sPESI»[All Fields] OR «HESTIA»[All Fields] OR «low risk»[All Fields] OR «low-risk»[All Fields] OR «right ventricular dysfunction»[All Fields] OR «echocardiograph*»[All Fields] OR «risk stratification»[All Fields] OR «predict*»[All Fields]) AND («2008/10/30»[PDAT]: «3000/12/31»[PDAT]), with no restriction for languages. The systematic review was completed by a manual search of the literature and the investigators’ files.
Selection Criteria and Outcome MeasuresThe meta-analysis included studies that met the following selection criteria: (1) retrospective or prospective observational studies; (2) population: patients with an objective diagnosis of low-risk acute symptomatic PE according to a validated prognostic scale (i.e., PESI, simplified PESI, Hestia criteria); (3) intervention: transthoracic echocardiography performed during acute phase PE; (4) outcome variables: early all-cause death (i.e., in the first 30 days) as primary variable, and early death due to PE as secondary variable.
Articles that did not include death as an outcome variable or which did not provide accurate data for evaluation of mortality were excluded. For duplicate publications, only the most recent was included.
Data Extraction and Quality AssessmentFor each study, 2 investigators (IA and DJ) extracted data referring to the baseline characteristics of the patients, echocardiography results, and number of events for each group. Discrepancies were resolved with the help of a third researcher (EM). The Quality in Prognosis Studies tool was used to evaluate the quality of the studies selected.14
Statistical analysisThe meta-analysis was conducted in accordance with Meta-analysis of Observational Studies in Epidemiology (MOOSE) recommendations.15 Data were analyzed using Review Manager (RevMan) 5.3.5 software (Cochrane Collaboration, Copenhagen), which offers open-source statistical packages for meta-analysis. A random effects model was used for data aggregation, and the odds ratio (OR) and corresponding 95% confidence intervals (95% CI) were used in the summary. Heterogeneity of the original studies was assessed using the I statistic.2 Significance levels in all analyses were 2-tailed and P values <0.05 were considered significant. Publication bias was addressed using Begg's rank correlation test.
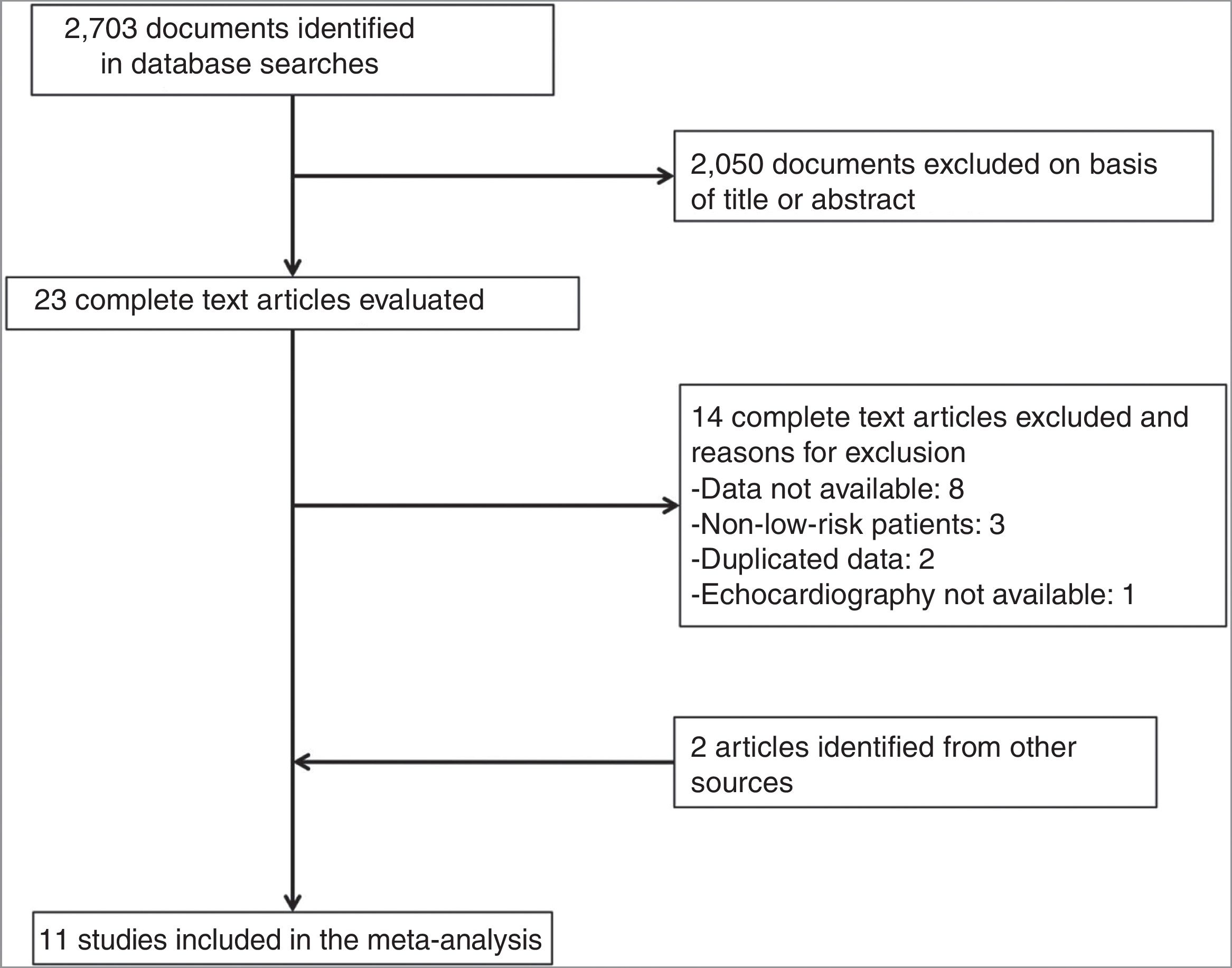
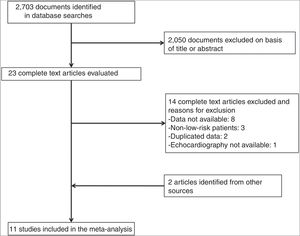
ResultsDescription of the StudiesOf 2073 articles evaluated, 23 were reviewed in depth and 6 were excluded. Two studies were added after the manual search, so a total of 19 studies met the inclusion criteria (Fig. 1). For 8 of these studies,16–23 the number of events in each group was obtained by contacting the corresponding authors. Eight studies were excluded because the authors did not provide the necessary data.24–31 Finally, we included 11 studies with 1868 patients with low-risk acute PE who underwent transthoracic echocardiography.16–23,32–34
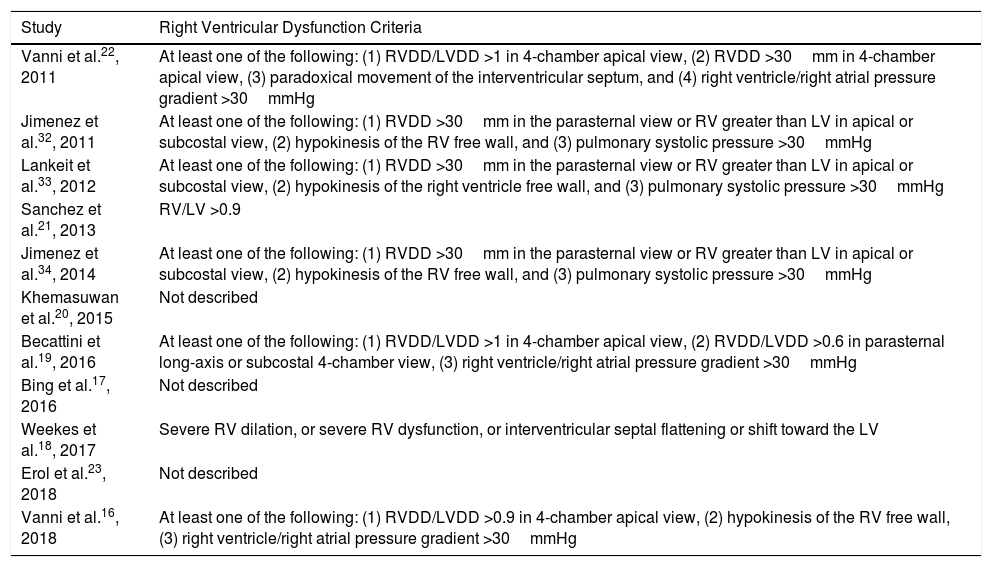
Eight studies were prospective16,19,21–23,32–34 and 3 were retrospective.17,18,20 Age and sex of study patients with a diagnosis of PE were similar in all studies (Table 1). Follow-up ranged from duration of hospital stay17,18,20,22,23 and 30 days after diagnosis of PE.16,19,21,32–34 The prevalence of echocardiographic RV dysfunction ranged between 11%21 and 58%.20 Echocardiographic criteria for RV dysfunction are shown in Table 2.
Description of Studies Included.
| Author | Year | Design | Event Evaluated | Clinical Scale | RV Dysfunction | Low-Risk Patients, n (%) | Sex | Age, Mean (SD) or Range, Years | Follow-up | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Vanni et al.22 | 2011 | Prospective | All-cause death | PESI | 48% | 145 (28) | 56% women | – | In-hospital | 4.3% mortality for patients with RV dysfunction vs. 0% for patients without RV dysfunction. |
| Jimenez et al.32 | 2011 | Prospective | PE death | PESI | 19% | 199 (34) | 57% women | 74 (65–82) | 30 days | 0% mortality for patients with RV dysfunction vs. 1.2% for patients without RV dysfunction. |
| Lankeit et al.33 | 2012 | Prospective | All-cause death | Simplified PESI | 34% | 165 (31) | 57% women | 74 (61–80) | 30 days | 0% mortality for patients with RV dysfunction vs. 0% for patients without RV dysfunction. |
| Sanchez et al.21 | 2013 | Prospective | All-cause death, cardiogenic shock, or thrombotic recurrence | PESI | 11% | 329 (56) | 53% women | 67 (52–77) | 30 days | 0% mortality for patients with RV dysfunction vs. 0.7% for patients without RV dysfunction. |
| Jimenez et al.34 | 2014 | Prospective | Complicated clinical course | Simplified PESI | 13% | 313 (37%) | 51% women | 72 (59–80) | 30 days | 2.5% mortality for patients with RV dysfunction vs. 0.7% for patients without RV dysfunction. |
| Khemasuwan et al.20 | 2015 | Retrospective | All-cause death | Simplified PESI | 58% | 92 (44) | 51% women | 61 (15) | In-hospital | 7.5% mortality for patients with RV dysfunction vs. 10% for patients without RV dysfunction. |
| Becattini et al.19 | 2016 | Prospective | All-cause death | Simplified PESI | 34% | 186 (21) | 54% women | 68 (16) | 30 days | 1.6% mortality for patients with RV dysfunction vs. 0% for patients without RV dysfunction. |
| Bing et al.17 | 2016 | Retrospective | All-cause death | Simplified PESI | 22% | 221 (15) | 56% women | 68 (16) | In-hospital | 2.1% mortality for patients with RV dysfunction vs. 0% for patients without RV dysfunction. |
| Weekes et al.18 | 2017 | Retrospective | All-cause death | Simplified PESI | 23% | 43 (35) | 49 | 59 (43–69) | In-hospital | 0% mortality for patients with RV dysfunction vs. 0% for patients without RV dysfunction. |
| Erol et al.23 | 2018 | Prospective | All-cause death | Simplified PESI | 21% | 33 (30) | 58% women | 47 | In-hospital | 0% mortality for patients with RV dysfunction vs. 0% for patients without RV dysfunction. |
| Vanni et al.16 | 2018 | Prospective | All-cause death | Simplified PESI | 17% | 100 (18) | 54% women | 76 (65–83) | 30 days | 4.3% mortality for patients with RV dysfunction vs. 0% for patients without RV dysfunction. |
PESI: Pulmonary Embolism Severity Index; RV: right ventricle; SD: standard deviation.
Right Ventricular Dysfunction Criteria.
| Study | Right Ventricular Dysfunction Criteria |
|---|---|
| Vanni et al.22, 2011 | At least one of the following: (1) RVDD/LVDD >1 in 4-chamber apical view, (2) RVDD >30mm in 4-chamber apical view, (3) paradoxical movement of the interventricular septum, and (4) right ventricle/right atrial pressure gradient >30mmHg |
| Jimenez et al.32, 2011 | At least one of the following: (1) RVDD >30mm in the parasternal view or RV greater than LV in apical or subcostal view, (2) hypokinesis of the RV free wall, and (3) pulmonary systolic pressure >30mmHg |
| Lankeit et al.33, 2012 | At least one of the following: (1) RVDD >30mm in the parasternal view or RV greater than LV in apical or subcostal view, (2) hypokinesis of the right ventricle free wall, and (3) pulmonary systolic pressure >30mmHg |
| Sanchez et al.21, 2013 | RV/LV >0.9 |
| Jimenez et al.34, 2014 | At least one of the following: (1) RVDD >30mm in the parasternal view or RV greater than LV in apical or subcostal view, (2) hypokinesis of the RV free wall, and (3) pulmonary systolic pressure >30mmHg |
| Khemasuwan et al.20, 2015 | Not described |
| Becattini et al.19, 2016 | At least one of the following: (1) RVDD/LVDD >1 in 4-chamber apical view, (2) RVDD/LVDD >0.6 in parasternal long-axis or subcostal 4-chamber view, (3) right ventricle/right atrial pressure gradient >30mmHg |
| Bing et al.17, 2016 | Not described |
| Weekes et al.18, 2017 | Severe RV dilation, or severe RV dysfunction, or interventricular septal flattening or shift toward the LV |
| Erol et al.23, 2018 | Not described |
| Vanni et al.16, 2018 | At least one of the following: (1) RVDD/LVDD >0.9 in 4-chamber apical view, (2) hypokinesis of the RV free wall, (3) right ventricle/right atrial pressure gradient >30mmHg |
LV: left ventricle; LVDD: left ventricular end-diastolic diameter; RV: right ventricle; RVDD: right ventricular end-diastolic diameter.
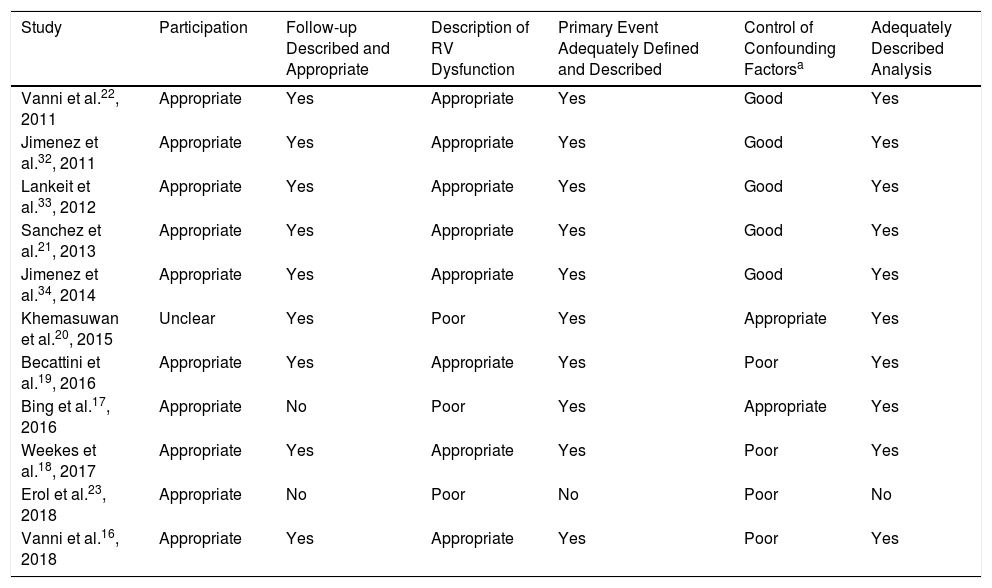
With regard to the quality of the studies included (Table 3), the participation and baseline characteristics of the patients were described adequately in most of the studies.16–19,21–23,32–34 All studies provided an adequate description of the RV dysfunction criteria used, with the exception of Khemasuwan et al., Bing et al. and Erol et al. Adjustments were made for potential confounding factors in 7 of the studies included.17,20–22,32–34 Events were adjudicated by a committee in only 3 of the studies.16,21,34
Quality of Studies Included.
| Study | Participation | Follow-up Described and Appropriate | Description of RV Dysfunction | Primary Event Adequately Defined and Described | Control of Confounding Factorsa | Adequately Described Analysis |
|---|---|---|---|---|---|---|
| Vanni et al.22, 2011 | Appropriate | Yes | Appropriate | Yes | Good | Yes |
| Jimenez et al.32, 2011 | Appropriate | Yes | Appropriate | Yes | Good | Yes |
| Lankeit et al.33, 2012 | Appropriate | Yes | Appropriate | Yes | Good | Yes |
| Sanchez et al.21, 2013 | Appropriate | Yes | Appropriate | Yes | Good | Yes |
| Jimenez et al.34, 2014 | Appropriate | Yes | Appropriate | Yes | Good | Yes |
| Khemasuwan et al.20, 2015 | Unclear | Yes | Poor | Yes | Appropriate | Yes |
| Becattini et al.19, 2016 | Appropriate | Yes | Appropriate | Yes | Poor | Yes |
| Bing et al.17, 2016 | Appropriate | No | Poor | Yes | Appropriate | Yes |
| Weekes et al.18, 2017 | Appropriate | Yes | Appropriate | Yes | Poor | Yes |
| Erol et al.23, 2018 | Appropriate | No | Poor | No | Poor | No |
| Vanni et al.16, 2018 | Appropriate | Yes | Appropriate | Yes | Poor | Yes |
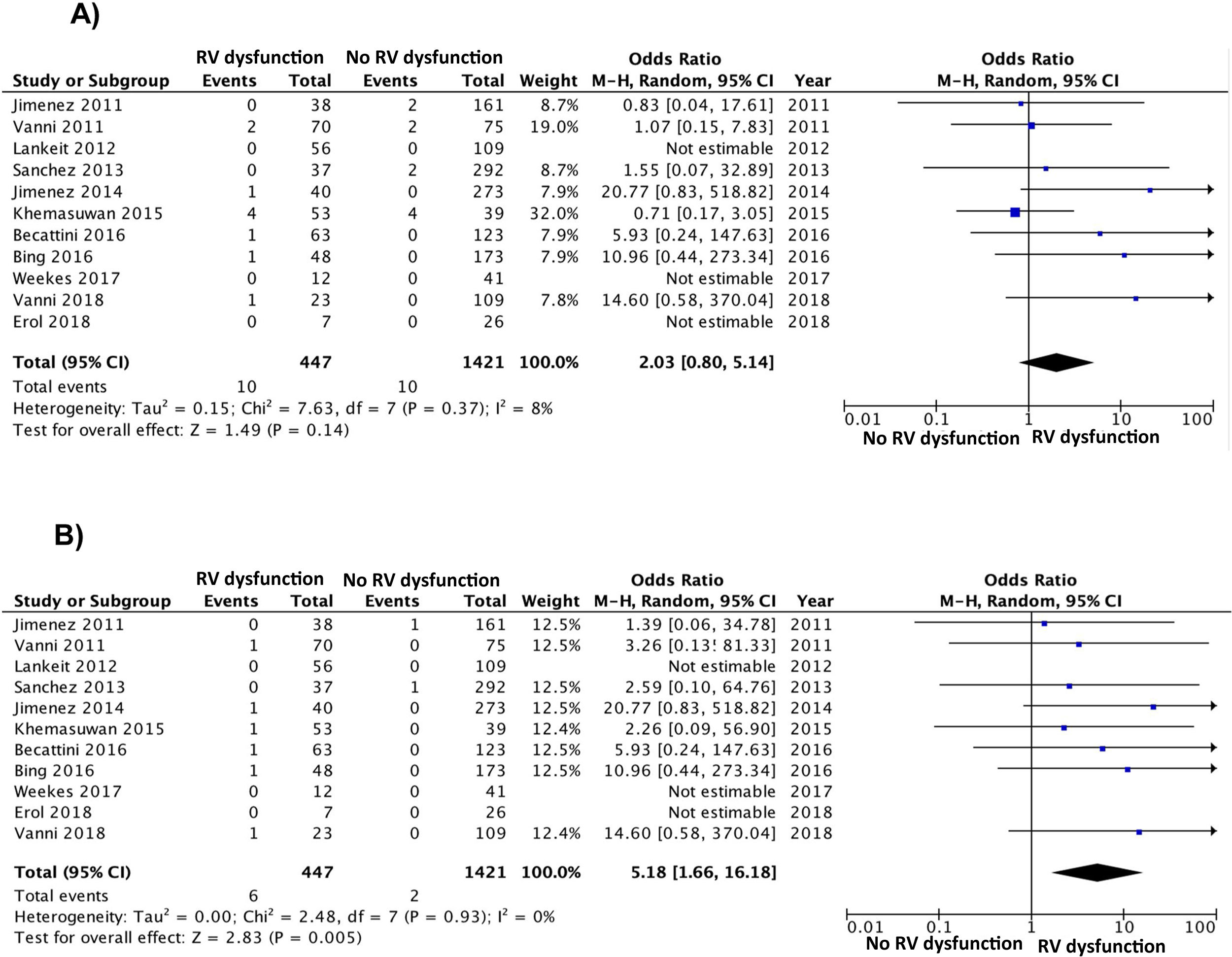
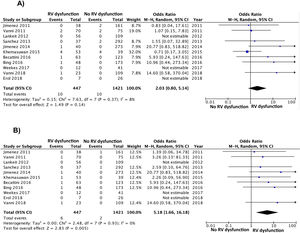
Of the 11 cohorts with 1868 patients with low-risk PE, 447 (24%; 95% CI, 22%–26%) had echocardiographic RV dysfunction, and 1421 did not (76%; 95% CI, 74%–78%). Ten of the 447 patients with low-risk PE and RV dysfunction died (2.2%; 95% CI, 1.1%–4.1%) compared to 10 of the 1421 patients without RV dysfunction (0.7%; 95% CI, 0.3%–1.3%).
Based on the results of the 11 observational studies, it was estimated that the combined OR of all-cause mortality of patients with RV dysfunction was 2.0 (95% CI, 0.8–5.1, P=.14; I2=8%, P=.37) (Fig. 2A). None of the studies showed statistically significant results. In all studies except for 2, patients with RV dysfunction had a worse, but not statistically significant, prognosis. No publication bias was revealed by Begg's rank correlation test.
All studies provided information on mortality due to PE in the short term. PE mortality was 1.3% (6 of 447 patients; 95% CI, 0.5%–2.9%) and 0.1% (2 of 1421 patients; 95% CI, 0.0%–0.5%) in patients with and without echocardiographic RV dysfunction, respectively. The combined OR of PE mortality of patients with RV dysfunction was 5.2 (95% CI, 1.7–16, P=.01; I2=0%, P=.93) (Fig. 2B). A transthoracic echocardiography would have to be performed in 83 patients with low-risk acute PE in order to identify 1 patient who will die due to PE in the first 30 days after diagnosis.
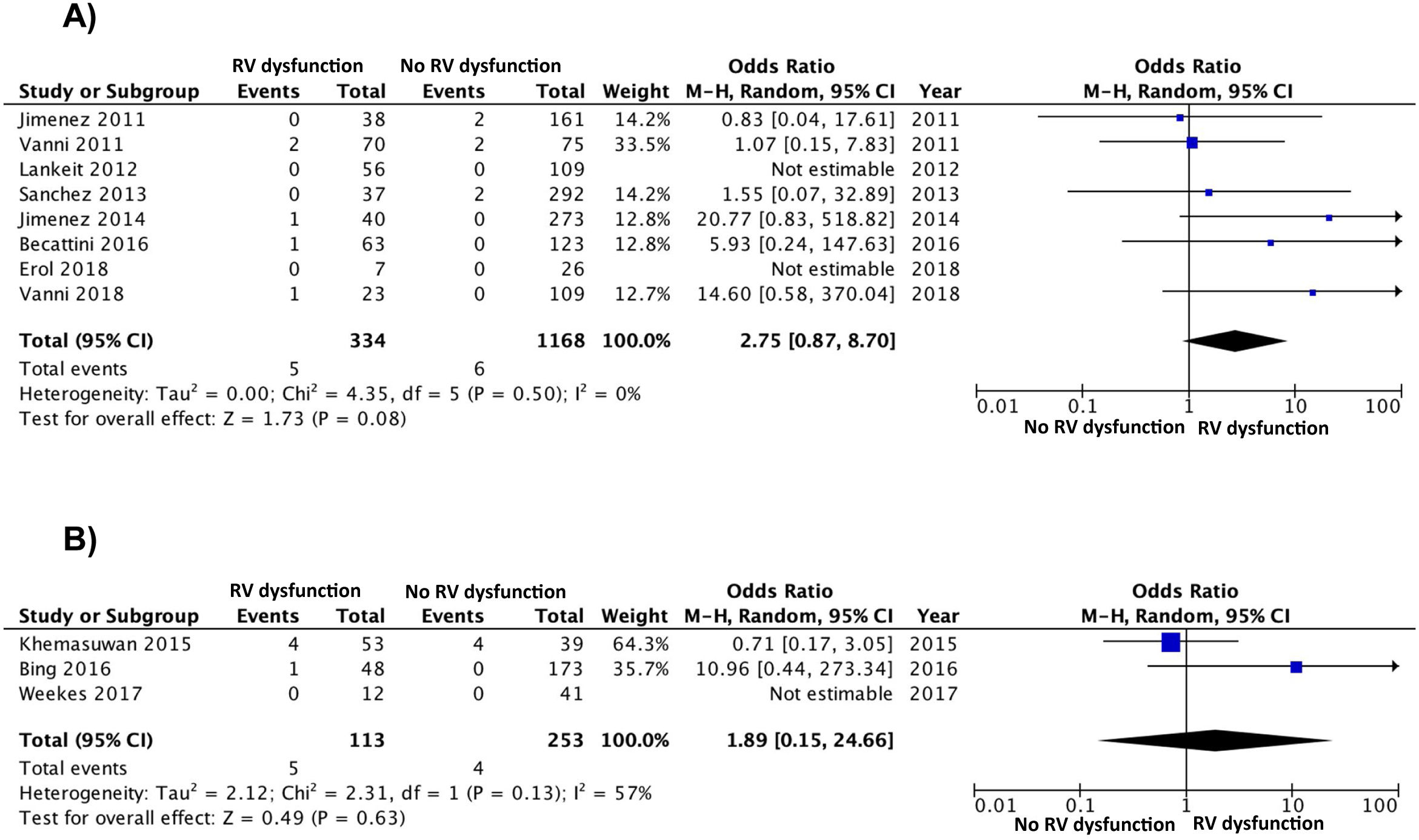
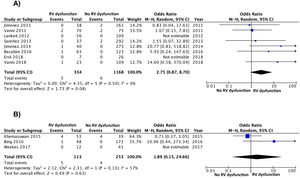
The results were similar when the analysis was limited to 8 studies (1502 patients) with a prospective design (OR 2.8; 95% CI, 0.9–8.7), with no clear heterogeneity (χ=4.4, df=5, P=.50, I2=0%) (Fig. 3A). The results were similar for the retrospective studies (OR 1.9; 95% CI, 0.2–25; heterogeneity χ=2.3, df=1, P=.13, I2=57%) (Fig. 3B). When the analysis was limited to the 8 studies that used the simplified PESI scale, the combined OR of mortality of patients with RV dysfunction was 4.4 (95% CI, 0.9–21, P=.07; I2=41%, P=.15) For studies that used the PESI scale, the OR was 1.1 (95% CI, 0.3–4.8, P=.90; heterogeneity χ=0.1, df=2, P=.96, I2=0%). A statistically significant association was maintained between RV dysfunction and PE mortality when the analysis was limited to 3 studies that had an adjudication committee of events (OR 9.2; 95% CI, 1.4–59, P=.02; heterogeneity χ=0.9, df=2, P=.63, I2=0%).
DiscussionThis study evaluated the prognostic utility of transthoracic echocardiography in patients with low-risk acute symptomatic PE. There were no statistically significant differences in all-cause mortality among low-risk patients with or without echocardiographic RV dysfunction. However, the presence of echocardiographic RV dysfunction resulted in a 5-fold increase in risk of PE death in the short term.
Risk stratification for clinical events is a basic variable in the management of patients with acute symptomatic pulmonary embolism. Clinical prognostic models were developed to identify PE patients with a low risk of complications, who might benefit from shorter hospital stays or even ambulatory treatment of their disease. The results of previous meta-analyses indicate that, in general, clinical scales reliably identify PE patients with a low risk of mortality in the first 30 days after diagnosis.35 Even so, it has not been fully clarified whether transthoracic echocardiography should be routinely performed in this subgroup of patients.
What are the implications of these findings in clinical practice? Given that the results of our meta-analysis indicate that echocardiographic RV dysfunction is not associated with all-cause mortality, and that in many centers this procedure is not always available, it seems reasonable not to routinely request this examination in patients with low-risk acute symptomatic PE. However, performing it early (i.e., in the first hours after diagnosis) might be useful for low-risk PE patients seen in centers with short stay programs and/or home treatment of PE, since our results indicate that the risk of PE death is negligible if echocardiographic RV dysfunction can be ruled out.
In a previous meta-analysis, Barco et al. found that RV dysfunction conferred a 4-fold increase in the risk of early all-cause mortality in patients with low-risk PE (according to a validated clinical prognostic scale).11 There are several reasons for this discrepancy with our study. These authors, unlike us, included data from studies that used angio-CT scan of the chest or echocardiography indistinctly to evaluate RV size/function. Moreover, our systematic review identified 8 studies with 1726 patients who were not included in the meta-analysis.
Our study has some limitations. Firstly, we were not able to adjust the association between RV dysfunction and mortality due to various confounding factors. This type of adjustment could be performed in a meta-analysis of individual data. Secondly, the validity of the results and the conclusions of the meta-analysis depend on the quality of the individual studies, so the combination of biased studies could have exaggerated this bias even further. Thirdly, there is no consensus in the literature for the definition of echocardiographic RV dysfunction. However, the studies included used very similar definitions, and given the fact that most studies found a worse (while not significant) prognosis in patients with RV dysfunction, it seems unlikely that this limitation could have influenced the results of this meta-analysis. Finally, only 3 of the studies had an events adjudication committee, which could explain the difference between the results for the primary variable (all-cause mortality) and the secondary variable (PE mortality). However, similar findings were obtained when the analyses were restricted to these studies, thus reinforcing the validity of our results.
In conclusion, in patients with low-risk PE according to a clinical prognostic scale, echocardiographic RV dysfunction is not associated with an increased risk of premature death from any cause. Since the presence of echocardiographic RV dysfunction was significantly associated with PE death, it seems reasonable to request this procedure only in low-risk patients in whom outpatient treatment of their disease is contemplated. Well-designed studies are required in order to determine if the evaluation of RV using chest angio-CT scan can provide similar information to that of transthoracic echocardiography in this group of patients.
FundingThis study was partially funded by the Instituto de Salud Carlos III (PI15/00207).
Conflict of InterestsD. Jiménez was the principal investigator of 2 of the studies included in this meta-analysis.
We are grateful to Drs. Becattini, Erol, Khemasuwan, Ng, Sánchez, Vanni, and Weekes for providing the data to conduct this meta-analysis.
Please cite this article as: Andrade I, García A, Mercedes E, León F, Velasco D, Rodríguez C, et al. Necesidad de una ecocardiografía transtorácica en pacientes con tromboembolia de pulmón de riesgo bajo: revisión sistemática y metanálisis. Arch Bronconeumol. 2020;56:306–313.