Inhaled drugs are deposited directly in the respiratory tract. They therefore achieve higher concentrations with faster onset of action and fewer side effects than when used systemically. Nebulized drugs are mainly recommended for patients that require high doses of bronchodilators, when they need to inhale drugs that only exist in this form (antibiotics or dornase alfa) or when they are unable to use other inhalation devices. Technological development in recent years has led to new devices that optimize pulmonary deposits and reduce the time needed for treatment. In this review we focus solely on drugs currently used, or under investigation, for nebulization in adult patients, basically bronchodilators, inhaled steroids, antibiotics, antifungals, mucolytics and others such as anticoagulants, prostanoids, and lidocaine.
Los fármacos inhalados se depositan directamente en el tracto respiratorio, con lo que se alcanzan altas concentraciones, con un inicio de acción más rápido y con menores efectos secundarios que si se emplea la vía sistémica. Los fármacos nebulizados se recomiendan fundamentalmente en pacientes que requieren dosis altas de broncodilatadores, cuando precisan inhalar fármacos que solo pueden administrarse nebulizados (como los antibióticos o la dornasa alfa) y si no son capaces de utilizar otros dispositivos de inhalación. El desarrollo tecnológico de los últimos años ha permitido contar con dispositivos que optimizan el depósito pulmonar y disminuyen el tiempo necesario para realizar el tratamiento. En esta revisión nos ceñiremos únicamente a aquellos fármacos que se administran –o están en investigación– en nebulización en pacientes adultos; fundamentalmente a los broncodilatadores, corticoides inhalados, antibióticos, antifúngicos, mucolíticos y otros como los prostanoides, los anticoagulantes o la lidocaína.
The inhaled route has been used for centuries to administer various substances and drugs. Inhaled drugs are deposited directly in the respiratory tract, and therefore achieve higher concentrations with faster onset of action and fewer side effects than when used systemically. The 3 modalities commonly used are pressurized metered-dose inhalers, dry powder inhalers, and nebulizers. As a general rule, nebulizers are not recommended if the drug can be administered using other devices.1–4 The European Respiratory Society2 recommends them for patients who require high doses of bronchodilators, when they need to inhale drugs that only exist in this form (antibiotics or dornase alfa), or when they are unable to use other inhalation devices.2
Medicinal products for inhalation are developed with specific characteristics that are different to their systemically administered analogs. The efficacy of nebulization depends on many factors, including the characteristics of the medicinal product (size, shape, density, and surface tension of the particle), the anatomy of the airway, the patient's inhalation technique, and the nebulization system.1 The size of the particles produced by a nebulizer depends on the properties of the solution and the flow rate. The higher the flow rate, the smaller the size of the particles in the aerosol. Particles of between 1 and 5μm are most likely to reach the correct sites in the bronchial tree and achieve the sought-after therapeutic effect.1–4 Nebulization devices or systems are composed of a nebulization chamber containing the liquid to be nebulized and from which the aerosol is generated, and an energy source to produce the mist. There are 3 types of nebulizers for clinical use: ultrasonic, jet (pneumatic) and mesh. Their main characteristics are summarized in Table 1. Nebulized drugs should preferably be administered using the nebulizers with which the clinical trials were conducted. Patients should be trained in their use, cleaning and maintenance. Technological development in recent years has led to new devices that optimize lung deposition and reduce the time needed to administer the treatment.1,4,5 In this review we will focus on currently available or investigational drugs for use in nebulization in adult patients.
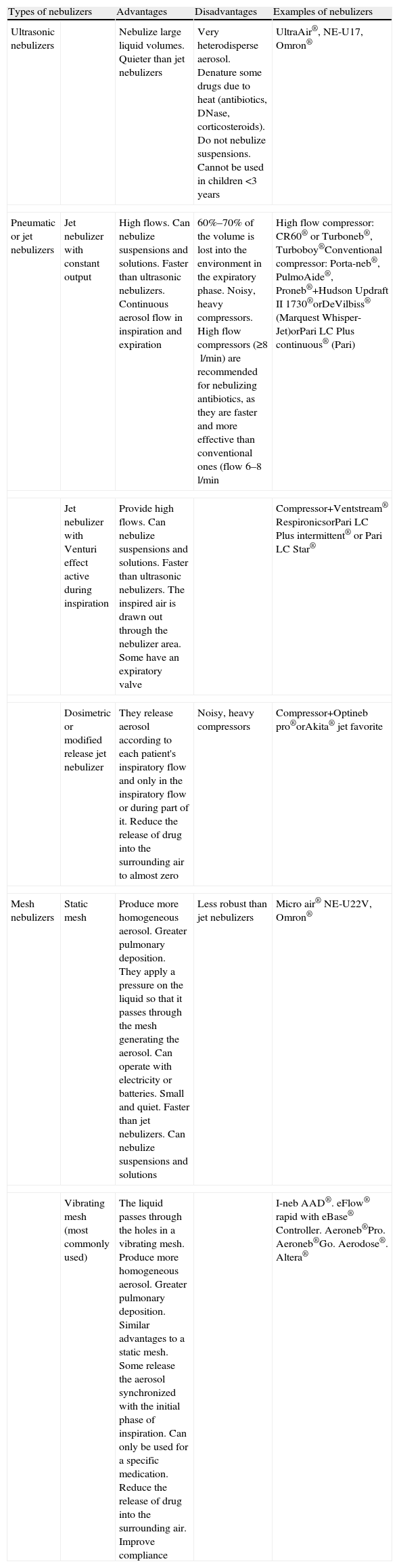
Types of Nebulizers and their Characteristics, Advantages and Disadvantages.
| Types of nebulizers | Advantages | Disadvantages | Examples of nebulizers | |
| Ultrasonic nebulizers | Nebulize large liquid volumes. Quieter than jet nebulizers | Very heterodisperse aerosol. Denature some drugs due to heat (antibiotics, DNase, corticosteroids). Do not nebulize suspensions. Cannot be used in children <3 years | UltraAir®, NE-U17, Omron® | |
| Pneumatic or jet nebulizers | Jet nebulizer with constant output | High flows. Can nebulize suspensions and solutions. Faster than ultrasonic nebulizers. Continuous aerosol flow in inspiration and expiration | 60%–70% of the volume is lost into the environment in the expiratory phase. Noisy, heavy compressors. High flow compressors (≥8l/min) are recommended for nebulizing antibiotics, as they are faster and more effective than conventional ones (flow 6–8l/min | High flow compressor: CR60® or Turboneb®, Turboboy®Conventional compressor: Porta-neb®, PulmoAide®, Proneb®+Hudson Updraft II 1730®orDeVilbiss® (Marquest Whisper-Jet)orPari LC Plus continuous® (Pari) |
| Jet nebulizer with Venturi effect active during inspiration | Provide high flows. Can nebulize suspensions and solutions. Faster than ultrasonic nebulizers. The inspired air is drawn out through the nebulizer area. Some have an expiratory valve | Compressor+Ventstream® RespironicsorPari LC Plus intermittent® or Pari LC Star® | ||
| Dosimetric or modified release jet nebulizer | They release aerosol according to each patient's inspiratory flow and only in the inspiratory flow or during part of it. Reduce the release of drug into the surrounding air to almost zero | Noisy, heavy compressors | Compressor+Optineb pro®orAkita® jet favorite | |
| Mesh nebulizers | Static mesh | Produce more homogeneous aerosol. Greater pulmonary deposition. They apply a pressure on the liquid so that it passes through the mesh generating the aerosol. Can operate with electricity or batteries. Small and quiet. Faster than jet nebulizers. Can nebulize suspensions and solutions | Less robust than jet nebulizers | Micro air® NE-U22V, Omron® |
| Vibrating mesh (most commonly used) | The liquid passes through the holes in a vibrating mesh. Produce more homogeneous aerosol. Greater pulmonary deposition. Similar advantages to a static mesh. Some release the aerosol synchronized with the initial phase of inspiration. Can only be used for a specific medication. Reduce the release of drug into the surrounding air. Improve compliance | I-neb AAD®. eFlow® rapid with eBase® Controller. Aeroneb®Pro. Aeroneb®Go. Aerodose®. Altera® | ||
Various systematic reviews have shown that the 3 types of devices commonly used to administer inhaled bronchodilators and corticosteroids (pressurized inhalers, dry powder inhalers, and nebulizers) have a similar efficacy when used correctly.2–4,6,7 However, clinical practice has shown that some patients (particularly the elderly) with physical or mental limitations, or with serious disease, are unable to correctly use dry powder or pressurized metered-dose inhalers.8 These patients, together with those who prefer nebulizers to other inhalers, could benefit from nebulized drug delivery.2,3,9–13
Short-acting bronchodilators are those most often used in nebulization, with drugs such as salbutamol and ipratropium bromide being currently available. Their combined use has been found to obtain a 24% improvement in the FEV1 compared to salbutamol alone, and a 37% improvement with respect to ipratropium bromide alone, in patients with chronic obstructive pulmonary disease (COPD).14 This treatment can improve quality of life even with concomitant use of another inhaler.15 Formoterol is the only long-acting bronchodilator available (as formoterol fumarate or arformoterol), although not in Spain. Various studies have demonstrated its effectiveness in the treatment of patients with COPD.16–18
Nebulized corticosteroids,19–28 principally budesonide20–23 (although also flunisolide,24 fluticasone,25 and beclomethasone26,27), can be considered an effective alternative in patients with asthma or COPD who are unwilling or unable to use other inhalation devices (Table 2). In a study of elderly patients with asthma or COPD who found it difficult to use other devices, Marcus et al.28 observed less use of systemic corticosteroids and fewer visits to the emergency department when long term treatment was maintained with nebulized corticosteroids. Maltais et al.22 compared the efficacy of nebulized budesonide and systemic corticosteroids in patients with COPD exacerbations and found no differences in FEV1 improvement, hospital stay, or adverse effects. The use of higher or more frequent doses could be a safe alternative to systemic corticosteroids, without their accompanying side effects.23
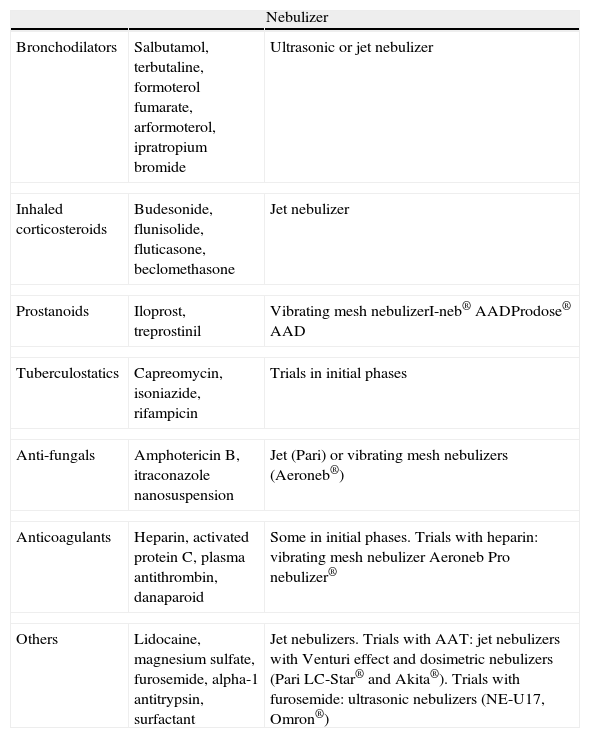
Different Types of Drugs for Nebulization.
| Nebulizer | ||
| Bronchodilators | Salbutamol, terbutaline, formoterol fumarate, arformoterol, ipratropium bromide | Ultrasonic or jet nebulizer |
| Inhaled corticosteroids | Budesonide, flunisolide, fluticasone, beclomethasone | Jet nebulizer |
| Prostanoids | Iloprost, treprostinil | Vibrating mesh nebulizerI-neb® AADProdose® AAD |
| Tuberculostatics | Capreomycin, isoniazide, rifampicin | Trials in initial phases |
| Anti-fungals | Amphotericin B, itraconazole nanosuspension | Jet (Pari) or vibrating mesh nebulizers (Aeroneb®) |
| Anticoagulants | Heparin, activated protein C, plasma antithrombin, danaparoid | Some in initial phases. Trials with heparin: vibrating mesh nebulizer Aeroneb Pro nebulizer® |
| Others | Lidocaine, magnesium sulfate, furosemide, alpha-1 antitrypsin, surfactant | Jet nebulizers. Trials with AAT: jet nebulizers with Venturi effect and dosimetric nebulizers (Pari LC-Star® and Akita®). Trials with furosemide: ultrasonic nebulizers (NE-U17, Omron®) |
Nebulized antibiotics (NAB) (penicillin and streptomycin) were first used for the treatment of bronchial infection in the 1950s.29,30 These early attempts gave way to the use of a wider range of NABs, prepared from their intravenous formulation, essentially in cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa (PA) infection. They have also been used for the treatment of bronchial infection in non-CF bronchiectasis (NCFB), COPD, and in ventilator-associated pneumonia (VAP). All this has driven the commercialization of specific antibiotic preparations for nebulization (Table 3) and the launch of clinical trials with different antibiotics for inhalation (some in dry power form, which will not be discussed here) in different diseases.29–33 NABs should preferably be administered using the nebulizers with which the trials were conducted.1–4,33,34
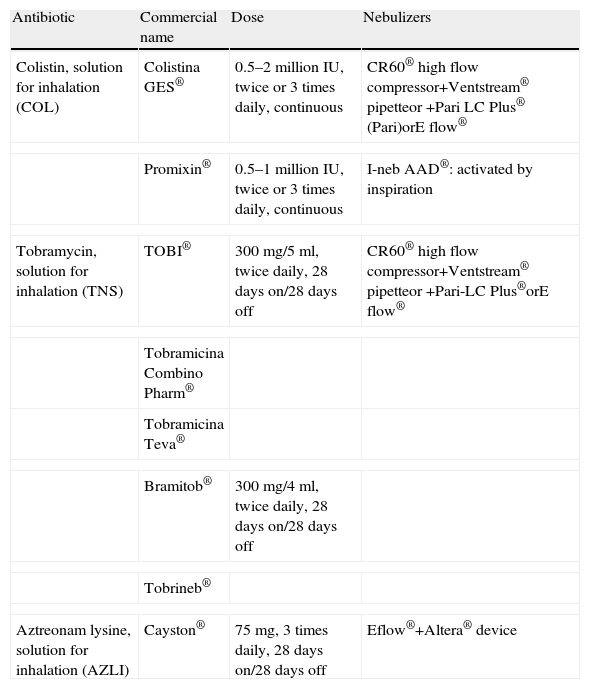
Antibiotics Marketed in Spain for Nebulized Use.
| Antibiotic | Commercial name | Dose | Nebulizers |
| Colistin, solution for inhalation (COL) | Colistina GES® | 0.5–2 million IU, twice or 3 times daily, continuous | CR60® high flow compressor+Ventstream® pipetteor +Pari LC Plus® (Pari)orE flow® |
| Promixin® | 0.5–1 million IU, twice or 3 times daily, continuous | I-neb AAD®: activated by inspiration | |
| Tobramycin, solution for inhalation (TNS) | TOBI® | 300mg/5ml, twice daily, 28 days on/28 days off | CR60® high flow compressor+Ventstream® pipetteor +Pari-LC Plus®orE flow® |
| Tobramicina Combino Pharm® | |||
| Tobramicina Teva® | |||
| Bramitob® | 300mg/4ml, twice daily, 28 days on/28 days off | ||
| Tobrineb® | |||
| Aztreonam lysine, solution for inhalation (AZLI) | Cayston® | 75mg, 3 times daily, 28 days on/28 days off | Eflow®+Altera® device |
The benefit of prompt treatment with NABs (tobramycin [TIS],35–38,40–42 colistin39,40,43 or aztreonam-lysine [AZLI]44) has been demonstrated in CF patients with early PA infection, as they achieve high rates of eradication and delay the onset of chronic bronchial infection.
Long-term administration of NABs has been shown to be effective in the treatment of chronic bronchial infection with PA in patients with CF.31,35–39,45–59 There are several options,35–38 including intermittent inhaled antibiotic therapy with 28 day treatment periods and 28 rest days using TIS31,45–49 or AZLI,38,55–59 or continuous treatment with colestin.36,39,50–52 Studies have already been conducted on other NABs not yet marketed in Spain, which have been shown to be effective and well-tolerated in patients with CF, such as levofloxacin,60,61 liposomal amikacin (Arikace®),32,62 and the combination fosfomycin+tobramycin.63Table 4 summarizes the main studies with NABs in patients with CF and chronic PA infection.
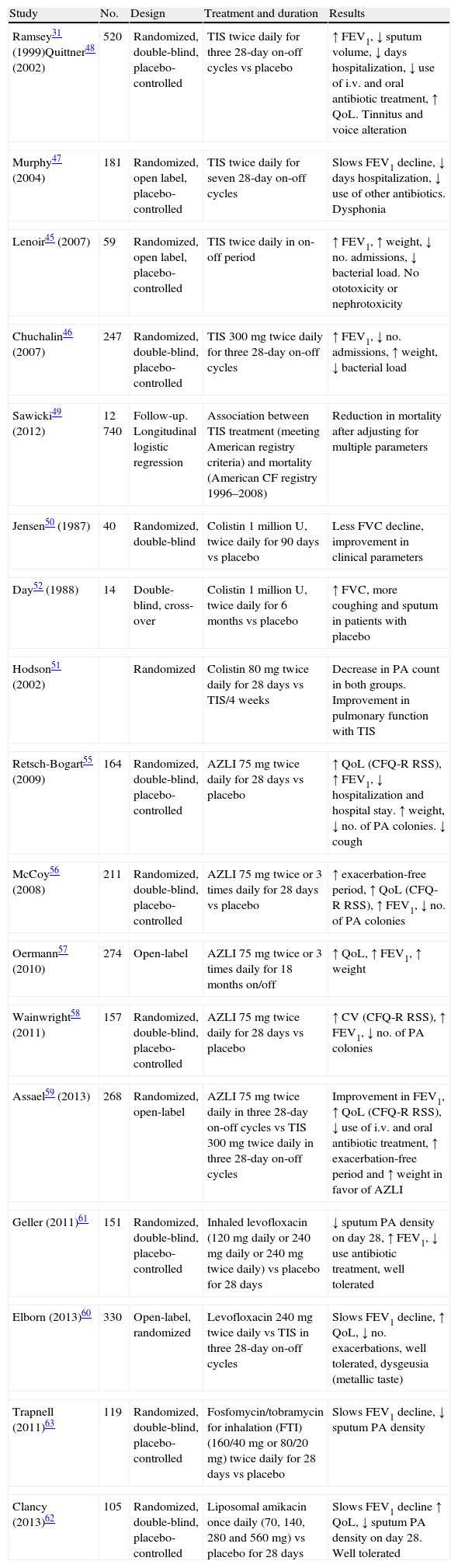
Studies on Nebulized Antibiotic Therapy in Patients With Cystic Fibrosis-related Bronchiectasis With Chronic Pseudomonas aeruginosa Infection.
| Study | No. | Design | Treatment and duration | Results |
| Ramsey31 (1999)Quittner48 (2002) | 520 | Randomized, double-blind, placebo-controlled | TIS twice daily for three 28-day on-off cycles vs placebo | ↑ FEV1, ↓ sputum volume, ↓ days hospitalization, ↓ use of i.v. and oral antibiotic treatment, ↑ QoL. Tinnitus and voice alteration |
| Murphy47 (2004) | 181 | Randomized, open label, placebo-controlled | TIS twice daily for seven 28-day on-off cycles | Slows FEV1 decline, ↓ days hospitalization, ↓ use of other antibiotics. Dysphonia |
| Lenoir45 (2007) | 59 | Randomized, open label, placebo-controlled | TIS twice daily in on-off period | ↑ FEV1, ↑ weight, ↓ no. admissions, ↓ bacterial load. No ototoxicity or nephrotoxicity |
| Chuchalin46 (2007) | 247 | Randomized, double-blind, placebo-controlled | TIS 300mg twice daily for three 28-day on-off cycles | ↑ FEV1, ↓ no. admissions, ↑ weight, ↓ bacterial load |
| Sawicki49 (2012) | 12740 | Follow-up. Longitudinal logistic regression | Association between TIS treatment (meeting American registry criteria) and mortality (American CF registry 1996–2008) | Reduction in mortality after adjusting for multiple parameters |
| Jensen50 (1987) | 40 | Randomized, double-blind | Colistin 1 million U, twice daily for 90 days vs placebo | Less FVC decline, improvement in clinical parameters |
| Day52 (1988) | 14 | Double-blind, cross-over | Colistin 1 million U, twice daily for 6 months vs placebo | ↑ FVC, more coughing and sputum in patients with placebo |
| Hodson51 (2002) | Randomized | Colistin 80mg twice daily for 28 days vs TIS/4 weeks | Decrease in PA count in both groups. Improvement in pulmonary function with TIS | |
| Retsch-Bogart55 (2009) | 164 | Randomized, double-blind, placebo-controlled | AZLI 75mg twice daily for 28 days vs placebo | ↑ QoL (CFQ-R RSS), ↑ FEV1, ↓ hospitalization and hospital stay. ↑ weight, ↓ no. of PA colonies. ↓ cough |
| McCoy56 (2008) | 211 | Randomized, double-blind, placebo-controlled | AZLI 75mg twice or 3 times daily for 28 days vs placebo | ↑ exacerbation-free period, ↑ QoL (CFQ-R RSS), ↑ FEV1, ↓ no. of PA colonies |
| Oermann57 (2010) | 274 | Open-label | AZLI 75mg twice or 3 times daily for 18 months on/off | ↑ QoL, ↑ FEV1, ↑ weight |
| Wainwright58 (2011) | 157 | Randomized, double-blind, placebo-controlled | AZLI 75mg twice daily for 28 days vs placebo | ↑ CV (CFQ-R RSS), ↑ FEV1, ↓ no. of PA colonies |
| Assael59 (2013) | 268 | Randomized, open-label | AZLI 75mg twice daily in three 28-day on-off cycles vs TIS 300mg twice daily in three 28-day on-off cycles | Improvement in FEV1, ↑ QoL (CFQ-R RSS), ↓ use of i.v. and oral antibiotic treatment, ↑ exacerbation-free period and ↑ weight in favor of AZLI |
| Geller (2011)61 | 151 | Randomized, double-blind, placebo-controlled | Inhaled levofloxacin (120mg daily or 240mg daily or 240mg twice daily) vs placebo for 28 days | ↓ sputum PA density on day 28, ↑ FEV1, ↓ use antibiotic treatment, well tolerated |
| Elborn (2013)60 | 330 | Open-label, randomized | Levofloxacin 240mg twice daily vs TIS in three 28-day on-off cycles | Slows FEV1 decline, ↑ QoL, ↓ no. exacerbations, well tolerated, dysgeusia (metallic taste) |
| Trapnell (2011)63 | 119 | Randomized, double-blind, placebo-controlled | Fosfomycin/tobramycin for inhalation (FTI) (160/40mg or 80/20mg) twice daily for 28 days vs placebo | Slows FEV1 decline, ↓ sputum PA density |
| Clancy (2013)62 | 105 | Randomized, double-blind, placebo-controlled | Liposomal amikacin once daily (70, 140, 280 and 560mg) vs placebo for 28 days | Slows FEV1 decline ↑ QoL, ↓ sputum PA density on day 28. Well tolerated |
AZLI, aztreonam lysine; CFQ-R RSS, Cystic Fibrosis Questionnaire-Revised; QoL, quality of life; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; PA, Pseudomonas aeruginosa; TIS, tobramycin solution for inhalation.
Exceptionally, other NABs are used in CF (prepared from their intravenous formulation) such as vancomycin for bronchial infection by methicillin-resistant Staphylococcus aureus (MRSA),64 or amikacin for treatment of rapidly growing atypical mycobacteria such as Mycobacterium abscessus65 or Mycobacterium avium complex66 as coadjuvant therapy and in combination with systemic antibiotics.
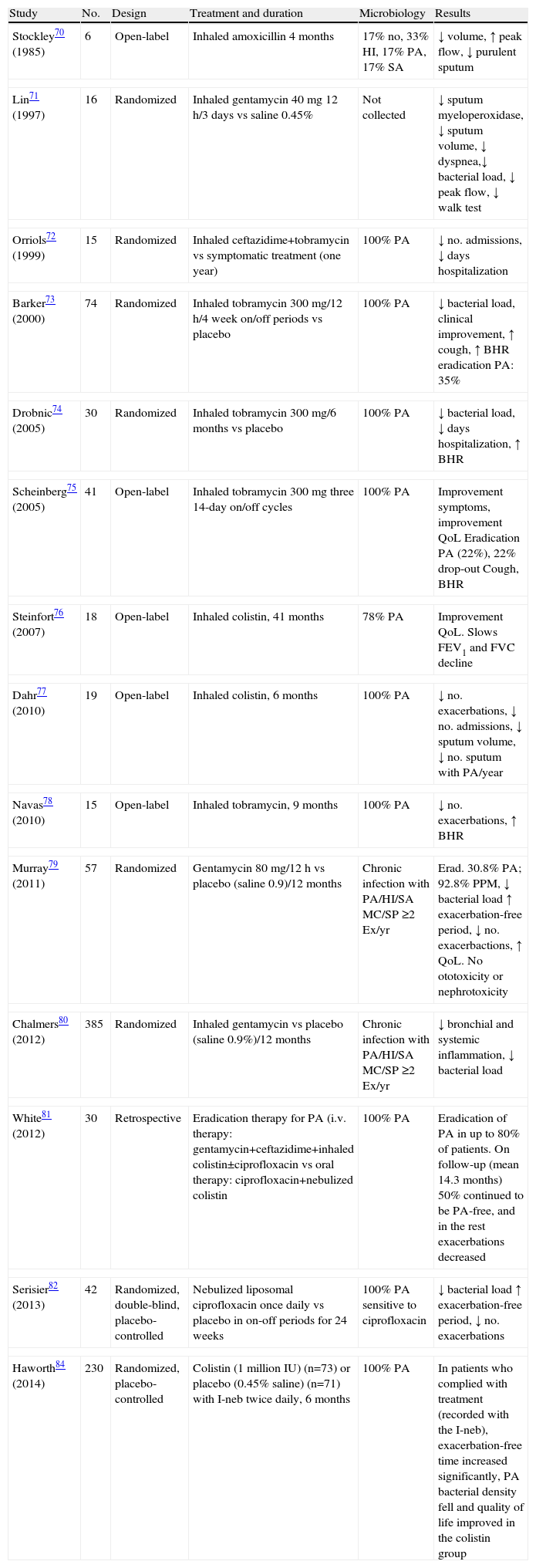
Nebulized Antibiotics in Non-cystic Fibrosis BronchiectasisThere is less evidence in NCFB, but studies conducted with different NABs have observed an improvement in quality of life and symptoms, with a reduction in sputum volume and purulence and exacerbations, a decrease in bronchial and systemic inflammation, and lower density of PA colonies and colonies of other microorganisms, with variable eradication rates.29,33,67–84Table 5 summarizes the main studies with NABs in patients with NCFB.
Studies on Nebulized Antibiotic Therapy in Patients With Non-cystic Fibrosis Bronchiectasis.
| Study | No. | Design | Treatment and duration | Microbiology | Results |
| Stockley70 (1985) | 6 | Open-label | Inhaled amoxicillin 4 months | 17% no, 33% HI, 17% PA, 17% SA | ↓ volume, ↑ peak flow, ↓ purulent sputum |
| Lin71 (1997) | 16 | Randomized | Inhaled gentamycin 40mg 12h/3 days vs saline 0.45% | Not collected | ↓ sputum myeloperoxidase, ↓ sputum volume, ↓ dyspnea,↓ bacterial load, ↓ peak flow, ↓ walk test |
| Orriols72 (1999) | 15 | Randomized | Inhaled ceftazidime+tobramycin vs symptomatic treatment (one year) | 100% PA | ↓ no. admissions, ↓ days hospitalization |
| Barker73 (2000) | 74 | Randomized | Inhaled tobramycin 300mg/12h/4 week on/off periods vs placebo | 100% PA | ↓ bacterial load, clinical improvement, ↑ cough, ↑ BHR eradication PA: 35% |
| Drobnic74 (2005) | 30 | Randomized | Inhaled tobramycin 300mg/6 months vs placebo | 100% PA | ↓ bacterial load, ↓ days hospitalization, ↑ BHR |
| Scheinberg75 (2005) | 41 | Open-label | Inhaled tobramycin 300mg three 14-day on/off cycles | 100% PA | Improvement symptoms, improvement QoL Eradication PA (22%), 22% drop-out Cough, BHR |
| Steinfort76 (2007) | 18 | Open-label | Inhaled colistin, 41 months | 78% PA | Improvement QoL. Slows FEV1 and FVC decline |
| Dahr77 (2010) | 19 | Open-label | Inhaled colistin, 6 months | 100% PA | ↓ no. exacerbations, ↓ no. admissions, ↓ sputum volume, ↓ no. sputum with PA/year |
| Navas78 (2010) | 15 | Open-label | Inhaled tobramycin, 9 months | 100% PA | ↓ no. exacerbations, ↑ BHR |
| Murray79 (2011) | 57 | Randomized | Gentamycin 80mg/12h vs placebo (saline 0.9)/12 months | Chronic infection with PA/HI/SA MC/SP ≥2 Ex/yr | Erad. 30.8% PA; 92.8% PPM, ↓ bacterial load ↑ exacerbation-free period, ↓ no. exacerbactions, ↑ QoL. No ototoxicity or nephrotoxicity |
| Chalmers80 (2012) | 385 | Randomized | Inhaled gentamycin vs placebo (saline 0.9%)/12 months | Chronic infection with PA/HI/SA MC/SP ≥2 Ex/yr | ↓ bronchial and systemic inflammation, ↓ bacterial load |
| White81 (2012) | 30 | Retrospective | Eradication therapy for PA (i.v. therapy: gentamycin+ceftazidime+inhaled colistin±ciprofloxacin vs oral therapy: ciprofloxacin+nebulized colistin | 100% PA | Eradication of PA in up to 80% of patients. On follow-up (mean 14.3 months) 50% continued to be PA-free, and in the rest exacerbations decreased |
| Serisier82 (2013) | 42 | Randomized, double-blind, placebo-controlled | Nebulized liposomal ciprofloxacin once daily vs placebo in on-off periods for 24 weeks | 100% PA sensitive to ciprofloxacin | ↓ bacterial load ↑ exacerbation-free period, ↓ no. exacerbations |
| Haworth84 (2014) | 230 | Randomized, placebo-controlled | Colistin (1 million IU) (n=73) or placebo (0.45% saline) (n=71) with I-neb twice daily, 6 months | 100% PA | In patients who complied with treatment (recorded with the I-neb), exacerbation-free time increased significantly, PA bacterial density fell and quality of life improved in the colistin group |
QoL, quality of life; Ex/yr, exacerbations/year; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; HI, Haemophilus influenzae; BHR, bronchial hyperresponsiveness; I-Neb, mesh nebulizer; MC, Moraxella catarrhalis; PPM, potentially pathogenic microorganisms; PA, Pseudomonas aeruginosa; SA, Staphylococcus aureus; SP, Streptococcus pneumoniae.
Two guidelines have so far been published, both of which essentially agree on the indications for NAB treatment in patients with NCFB. SEPAR guidelines33 recommend them in early colonization by PA (if eradication has not been achieved with oral ciprofloxacin), in chronic bronchial PA infection, and in chronic bronchial infection by other microorganisms when long-term oral antibiotic treatment is ineffective or there are adverse effects or resistances. British Thoracic Society guidelines67 recommend them in chronic PA infection when the patient has had more than 2 exacerbations in the previous year, or if they have significant morbidity.
Nebulized Antibiotics in Patients With Chronic Obstructive Pulmonary DiseasePatients with COPD can present persistent chronic bronchial infection, and a high prevalence of associated bronchiectasis has also been observed in those with moderate–severe disease, resulting in greater morbidity and poorer prognosis.85–88 According to Spanish COPD guidelines (GesEPOC),89 patients with the COPD frequent exacerbator phenotype with associated bronchiectasia and chronic bronchial infection are candidates for long-term or cyclical antibiotic treatment, and SEPAR Guidelines on bronchiectasia33 can be applied to them for the control of chronic bronchial infection. Several clinical trials have recently been set up to evaluate the safety, tolerance, and pharmacokinetics of formulations for inhaled use32 of levofloxacin90,91 and ciprofloxacin92 in patients with COPD, as well as their effectiveness in preventing exacerbations. Dal Negro et al.,93 in an uncontrolled study, investigated the effect of nebulized tobramycin (300mg/14 days) on the inflammatory markers in bronchial secretions in 13 patients with severe COPD colonized by multi-resistant PA, and obtained a significant reduction in markers of inflammation and, during the 6-month follow-up period, in bacteriological density and severe exacerbations. The study by Steinfort and Steinfort76 with nebulized colistin also included 4 patients with severe COPD chronically infected with multi-resistant Gram-negative bacteria; they observed an improvement in quality of life and a reduction in lung function decline.
Nebulized Antibiotics in Ventilator-associated PneumoniaSome studies have shown positive clinical results with NABs as adjuvant therapy for ventilator-associated pneumonia (VAP), with improved clinical severity scores, less microbial resistance and use of systemic antibiotics and/or fewer days of intubation.94–102 Studies range from the prevention of VAP96 to adjuvant therapy for the intravenous treatment of pneumonia caused by Gram-negative bacteria94 and the treatment of pneumonia caused by PA or multi-resistant Acinetobacter.97–101 Inhaled colistin has been shown to be effective as adjuvant therapy for the VAP fundamentally caused by multi-resistant pathogens (Acinetobacter and PA), with good bacteriological97–99 and clinical response.100,101
Palmer et al.,102 in a randomized, double blind placebo-controlled trial, included intubated patients who received NAB treatment according to Gram-stain (gentamycin 30mg every 8h for Gram-negative and vancomycin 120mg every 8h for Gram-positive) or placebo. NAB improved the clinical signs of pneumonia, reduced episodes of VAP, bacterial resistance, and the use of systemic antibiotics, and also facilitated weaning. In another randomized, placebo-controlled study,95 vancomycin in aerosol succeeded in eradicating the microorganism in 4 out of 5 patients with MRSA as the VAP causal agent. Although these cohorts were small and the results must be confirmed with larger trials, they suggest that aerosol therapy may be useful against MRSA.
The addition of antibiotics in aerosol form to systemic antibiotics may be considered in patients with multi-resistant microorganisms, in those who do not respond to systemic antibiotics, or in VAP.94–102
Pneumonia Caused by Pneumocystis jiroveciPentamidine in aerosol form is a relatively well-tolerated alternative to oral agents in the primary and secondary prevention of pneumonia caused by P. jiroveci in patients with HIV or other immunosuppressive states, such as hematopoietic stem cell transplant recipients.103
AntifungalsAspergillus fumigatus infection is the most common infection in lung transplant recipients. Inhaled amphotericin is the most common preventive strategy.104 It has good distribution at pulmonary level105 without modifying surfactant lipid levels,106 and has very low systemic absorption. It comes in 3 presentations: amphotericin B deoxycholate, amphotericin B lipid complex, and liposomal amphotericin B. The latter 2 are the most widely used as they are well tolerated and have better pulmonary distribution.105
Inhaled amphotericin for the prevention of invasive pulmonary aspergillosis has been used with good results in patients with hematological diseases with expected chemotherapy-induced neutropenia.107 It may also be an alternative to itraconazole or voriconazole treatment in patients with CF and allergic bronchopulmonary aspergillosis (ABPA). Proesmans et al.108 treated 7 patients with CF, ABPA, and difficulty in tapering steroids with amphotericin B deoxycholate or amphotericin B lipid complex, demonstrating its efficacy and safety, with improved lung function and only 1 treatment failure.108
The pharmacokinetic results of an aqueous suspension of itraconazole for patients with ABPA have recently been published, reporting high and long-lasting lung tissue concentrations, which enable once daily administration with minimal systemic exposure.109
Nebulized MucolyticsNebulized N-acetylcysteine has not been shown to be effective in COPD,10,89,110 CF,33,36,37,111 bronchiectasis,33,67,68 or conclusively in idiopathic pulmonary fibrosis.112 A recent randomized study by Homma et al.113 found that nebulized N-acetylcysteine monotherapy could be useful in patients with early stage idiopathic pulmonary fibrosis, because although there was no improvement in lung function, it did appear to halt deterioration.
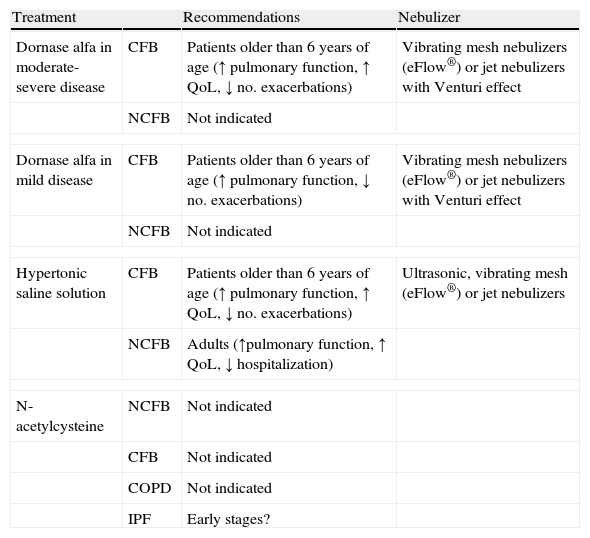
Inhalation of dornase alfa (DNase) has been shown to be clearly effective in CF,33,36,37,114 but in bronchiectasis due to other etiologies it may be ineffective or even harmful,115,116 and therefore is not recommended (Table 6).
Nebulized Mucolytics.
| Treatment | Recommendations | Nebulizer | |
| Dornase alfa in moderate-severe disease | CFB | Patients older than 6 years of age (↑ pulmonary function, ↑ QoL, ↓ no. exacerbations) | Vibrating mesh nebulizers (eFlow®) or jet nebulizers with Venturi effect |
| NCFB | Not indicated | ||
| Dornase alfa in mild disease | CFB | Patients older than 6 years of age (↑ pulmonary function, ↓ no. exacerbations) | Vibrating mesh nebulizers (eFlow®) or jet nebulizers with Venturi effect |
| NCFB | Not indicated | ||
| Hypertonic saline solution | CFB | Patients older than 6 years of age (↑ pulmonary function, ↑ QoL, ↓ no. exacerbations) | Ultrasonic, vibrating mesh (eFlow®) or jet nebulizers |
| NCFB | Adults (↑pulmonary function, ↑ QoL, ↓ hospitalization) | ||
| N-acetylcysteine | NCFB | Not indicated | |
| CFB | Not indicated | ||
| COPD | Not indicated | ||
| IPF | Early stages? | ||
CFB, cystic fibrosis-related bronchiectasis; NCFB, non-cystic fibrosis bronchiectasis; QoL, quality of life; IPF, idiopathic pulmonary fibrosis.
Inhalation of hypertonic saline solution (HSS) in patients with CF is effective, as it reduces exacerbations, improves quality of life, and slightly improves lung function.33,36,37,117
In patients with NCFB, HSS may reduce sputum viscosity and exacerbations, and improve quality of life and lung function.33,67,68 Nicolson et al.118 compared saline 6% with saline 0.9%, and found that both reduced colonization by microorganisms and exacerbations, while improving quality of life and lung function, with no significant differences between concentrations.118 HSS may also have immunomodulatory effects, and it has been observed that it may reduce interleukin-8 concentrations in sputum and bronchoalveolar lavage.119
Clinical trials are currently ongoing in COPD with HSS120 and BIO-11006 inhalation solution, which could have anti-inflammatory effects and inhibit mucous secretion.121
Other Nebulized Therapies (Table 2)Alpha-1 antitrypsin (AAT). The role of nebulized AAT as an anti-inflammatory treatment in CF is currently being investigated. In a randomized, double-blind study of 39 patients treated for 4 weeks with inhaled human recombinant AAT, the drug was well tolerated, but had a limited effect on inflammatory markers.122,123 In contrast, Griese et al.124 observed a decrease in the total PA load and in inflammatory markers in induced sputum with the inhalation of AAT. Nebulized l-arginine has recently been used in patients with CF,125 and was found to be safe and well-tolerated, increasing nitric acid production with no evidence of changes in bronchial inflammation.
Magnesium sulfate. Various studies have assessed the role of magnesium sulfate in asthma exacerbation. A recent Cochrane review did not find significant improvements when magnesium sulfate was added to beta-agonist treatment.126 In a subsequent meta-analysis, magnesium sulfate was added to usual treatment with corticosteroids and beta-2 agonists; in adults, this nebulized treatment was associated with a significant effect on pulmonary function and a reduction in hospital admissions.127 Goodacre et al.128 compared the effect of intravenous magnesium sulfate versus nebulized magnesium sulfate and placebo in 1084 adults, and found no benefit in adding it to standard treatment, either in dyspnea or in the rate of hospitalizations. There is currently no evidence that nebulized magnesium sulfate in adult patients has any effect on asthma exacerbation.126,129
Lidocaine. Lidocaine is a drug used as a local anesthetic and antiarrhythmic agent. In addition to its topical use to suppress coughing during fibrobronchoscopy, nebulized lidocaine has been used to treat difficult-to-control cough and asthma. Although it is not commercially available for nebulization, lidocaine hydrochloride solution for injection satisfies the requirements for use in nebulized form.130
Its use in refractory cough has been analyzed in various descriptive studies. However, the diversity of data with respect to dose, inhaled fraction according to the nebulizer used, comorbidities responsible for the cough, and previous treatment make it difficult to establish the ideal dose and to identify patients who could benefit from this treatment.130,131 In a recent study of 99 patients with difficult-to-control cough, using between 3 and 5ml of 4% lidocaine twice or 3 times a day, symptomatic control was achieved in 49% of the cases with no serious side effects.131 Therefore, although lidocaine is not a first choice treatment for persistent cough, it may be an alternative in patients who cannot tolerate or do not respond to other treatments.
Studies with nebulized lidocaine in asthma patients have not shown clear results; some have found an improvement in lung function and a reduction in the use of corticosteroids for symptom control,130,132 although others have not managed to reproduce these data,130,133 suggesting that further studies are required to consider this option in patients who require high doses of oral corticosteroids for symptom control.
Furosemide. This has also been used to relieve dyspnea via nebulized administration. It has been effective in patients with advanced cancer and severe dyspnea who do not respond to opiates. Various studies reviewing the effects of nebulized furosemide in patients with airway obstruction have found that it has a slight bronchodilator effect, or at least is capable of arresting bronchoconstriction.134 A recent randomized clinical trial of 100 patients with exacerbation of COPD in which the addition of inhaled furosemide to conventional treatment was compared, found a significant improvement in FEV1, dyspnea, pH, blood pressure and heart rate in the furosemide group.135
Prostanoids. In pulmonary arterial hypertension (PAH), there are 2 drugs within the prostanoid group that can be inhaled: iloprost and treprostinil.136 Iloprost is an analog of prostacyclin authorized in Spain as inhaled treatment in adults with PAH and functional class III. Although there was an initial improvement in functional grade, in the long-term only a few patients remained stable with iloprost in monotherapy.137 There are studies that support its efficacy in combination with bosentan and sildenafil, and also as an alternative to intravenous or subcutaneous prostanoids.132 Recent studies have explored the usefulness of iloprost in patients with acute respiratory distress syndrome (ARDS) and PAH, reporting improvement in gas exchange without any detriment to respiratory or hemodynamic parameters.138,139
Inhaled treprostinil was approved by the FDA in 2009 for patients with PAH and functional class III. Its effectiveness was initially demonstrated in patients who remained symptomatic despite treatment with bosentan and sildenafil,140 and it has also been used as an alternative to intravenous or subcutaneous prostanoids.141
Tuberculostatics. Some attempts have been made to treat multi-resistant tuberculosis via the inhaled route, such as with dry powder capreomycin, or formulations of various tuberculostatics such as liposomal capreomycin,142 isoniazide or rifampicin that have shown good levels via aerosol delivery in experimental animals.143 However, some characteristics of tuberculous lesions, such as the existence of poorly aerated areas or growth of microorganisms in biofilms, reduce the efficacy of nebulized therapy.
Anticoagulants. Impaired alveolar fibrin turnover is a fundamental aspect of severe pneumonia. Clinical studies suggest that natural coagulation inhibitors exert lung-protective effects via anticoagulant and possibly anti-inflammatory pathways. In experimental animals, the aerosolized administration of activated protein C, plasma anti-thrombin and heparin significantly reduced pulmonary coagulopathy, with no changes in systemic coagulation. Plasma anti-thrombin treatment inhibits the spread of S. pneumoniae and histopathological damage in the lung. It has not been possible to confirm this effect in pneumonia caused by PA. In a systematic review144 of preclinical and clinical trials on nebulized anticoagulants, only 3 clinical trials on nebulized heparin were identified.145–147 These found an improvement in survival in patients with acute lung injury associated with smoke inhalation, and also a reduction in the number of days on mechanical ventilation.
Surfactant. One meta-analysis148 that analyzed the administration of exogenous surfactant in ARDS found that it might improve oxygenation but not mortality. However, a wide variety of routes of administration were used in this meta-analysis, which concluded that the bronchoscopic route may be most promising, as the rate of pulmonary deposition using the nebulized route only reaches 4%–5%.149
ConclusionNebulized drugs are an effective therapeutic alternative in multiple respiratory diseases. Effective, rapid administration nebulization devices are currently available. The future will doubtless bring innovative drugs and new evidence that will allow us to resolve the many uncertainties that still exist.
Conflict of InterestCasilda Olveira has participated in expert committees and training activities promoted and funded by Chiesi, Gilead, Novartis and Praxis.
Adolfo Domenech has participated in training activities promoted and funded by Astra, Boehringer, Esteve, Glaxo, Novartis and Menarini.
Ana Muñoz has participated in training activities promoted and funded by Astra.
Please cite this article as: Olveira C, Muñoz A, Domenech A. Terapia nebulizada. Año SEPAR. Arch Bronconeumol. 2014;50:535–545.