Cystic fibrosis (CF) is the most common monogenic disease in the Caucasian population, and is potentially fatal.1,2 The major cause of morbidity and mortality is respiratory involvement, due to obstruction, inflammation and infection of the respiratory tract, which lead to epithelial damage, tissue remodeling, and terminal pulmonary disease.1,2 In recent decades, the most challenging infectious complications in CF have been caused by non-tuberculous mycobacteria (NTMB).3 These pathogens can cause significant lung disease, and may be difficult to diagnose in CF patients, due to overlapping symptoms, signs, and radiological findings. Moreover, the treatment of these infections constitutes an additional burden for patients.3 We report the first 2 cases of pediatric CF patients with lung infection caused by Mycobacterium lentiflavum (M. lentiflavum).
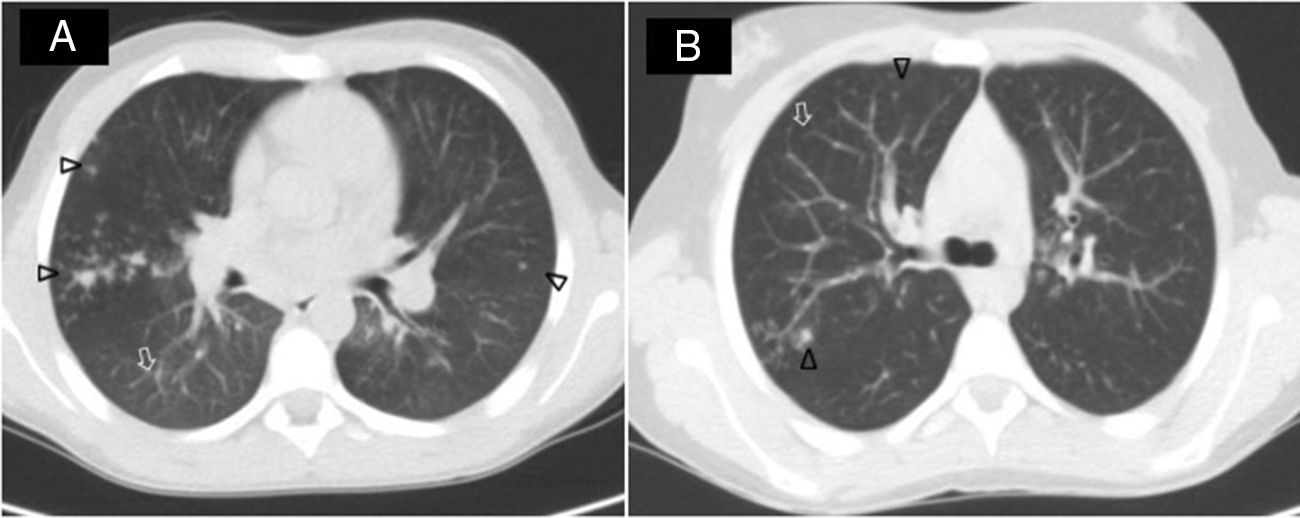
Our first case was a boy with CF with typical symptoms, 2 CFTR gene mutations (c.1521_1523delCTTT/1820_1903del), and 100 and 84mmol/l chloride in sweat tests. He was chronically colonized with oxacillin-sensitive Staphylococcus aureus and Haemophilus influenzae with intermittent Pseudomonas aeruginosa infection. At the age of 13 years, he experienced an increase in respiratory secretions, lung function decline, and weight loss. He was treated with ciprofloxacin, but showed no clinical improvement. Lung high-resolution computed tomography (HRCT) showed increased bronchiectasis with a tree-in-bud image and acinar nodules (Fig. 1A). After obtaining 2 sputum cultures positive for M. lentiflavum, the patient began treatment with clarithromycin (500mg/12h), cycloserine (500mg/24h), and ethambutol (500mg/24h) for 6 months, resulting in eradication of the pathogen.
Our second patient was an 11-year-old girl with a diagnosis of CF who presented persistent chronic cough, 102mmol/l chloride in 2 sweat tests, and 2 CFTR mutations (c.[1521_1523delCTT/c.1000C>T). She had intermittent colonization with oxacillin-sensitive S. aureus. The patient presented a slight increase in cough and worsening lung function, which coincided with the isolation of M. lentiflavum in 2 sputum samples. Lung HRCT showed pulmonary nodules and increased bronchiectasis (Fig. 1B). After 6 months of oral clarithromycin (500mg/12h), cycloserine 500mg/12h, ethambutol (500mg/24h), and nebulized amikacin, the patient's clinical and spirometric progress was good, and M. lentiflavum was eradicated.
In recent years, we have witnessed an increase in survival in CF for multiple reasons, including early diagnosis with neonatal screening, better nutritional support, earlier and more aggressive interventions for respiratory infections, multidisciplinary management, follow-up in reference units, and the development of cystic fibrosis transmembrane conductance regulators.1 Changes are also being observed in the epidemiology of pathogens associated with lung infections, with the emergence of microorganisms with a significant clinical impact that complicate the management of these patients and significantly affect lung function by perpetuating the vicious circle of inflammation and infection.4 One of these emerging pathogens is NTMB.2 These microorganisms are widely distributed in the environment, and the generally accepted mechanism of transmission is aerosolization,2,5 although evidence is emerging on transmission between humans, underlining the importance of strict adherence to protocols for the control of cross-infection.2 They can cause chronic lung infection, particularly in subjects with pre-existing inflammatory lung disease, such as our patients.2,5 The incidence increases with age,6 and the real prevalence, as defined by the American Thoracic Society, is not fully determined, although it is estimated to be between 4% and 14%.2Mycobacterium avium complex and Mycobacterium abscessus are the most frequently isolated NTMB.7,8
M. lentiflavum is a slow-growing, pigmented, scotocromogenic NTMB, first described in 1996.7–9 It grows at 25–37°C, forming colonies measuring 1–2mm in diameter, with bright yellow pigmentation.7,8,10 It rarely causes human infection; the most common form of presentation in children is cervical lymphadenitis,7,8,11,12 although infections have also been described in immunocompromised patients,7,8,11,12 and evidence is growing of cases of chronic lung disease in immunocompetent patients,7,12 such as the ones discussed here.
Because M. lentiflavum is an environmental microorganism, the clinical significance of lung infection caused by this bacterium in immunocompetent patients has been questioned.7,8,10,13 In our patients, the diagnosis of M. lentiflavum infection was made according to the criteria of the latest consensus of the European Society and American CF Foundation for the diagnosis of NTMB in CT patients. Specifically, both patients presented symptoms of respiratory exacerbation not attributable to other microorganisms or other causes, radiological worsening, and 2 sputum cultures positive for M. lentiflavum. These data were supported by the clear radiological, spirometric, and nutritional improvement and negative cultures obtained after the treatment. Given the clinical impact of infection by this pathogen on the lung disease of our patients, we suggest that M. lentiflavum is not always a simple contaminant, and we believe that it is essential to identify it in CF patients.9
The biochemical characteristics of this microorganism make it difficult to identify,9 as additional nucleic acid studies are required for its characterization: hsp-65 gene amplification, digestion with restriction enzymes and 16S rRNA amplification, with subsequent sequencing of a hypervariable region within the gene.9 The optimal therapeutic regimen has not been determined,10 but it is important to bear in mind that this micro-organism is resistant to most antituberculosis agents.12 Combinations of clarithromycin or azithromycin plus rifampicin, rifabutin or ethambutol9 are commonly used. Strains that are sensitive to amikacin, ciprofloxacin, cycloserine, canamycin, or ofloxacin have been identified.12 In our cases, eradication was achieved after 6 months of oral treatment with clarithromycin, cycloserine, and nebulized amikacin, and tolerance was good. Ophthalmological and audiometric studies are unchanged.
To conclude, we propose the inclusion of M. lentiflavum in the growing list of NTMB that cause lung disease in both immunocompromised and immunocompetent patients. It is essential to correctly identify this pathogen using specific molecular tools, and to determine its sensitivity profile, given the high rate of antimicrobial resistance. Clinical trials and/or studies are needed to clarify its real prevalence, to identify risk factors related with this infection, and to study the clinical impact in patients with chronic lung disease, specifically in CF. These steps will help improve diagnostic strategies and will support therapeutic decisions with consistent scientific evidence, in order to improve control of the infection and avoid the lung function decline associated with this pathogen.
Please cite this article as: Moreno Ortega M, Quintana Gallego E, Carrasco Hernández L, Pérez Borrego E, Delgado Pecellín I. Mycobacterium lentiflavum en pacientes con fibrosis quística: ¿colonizante o patogénico? Arch Bronconeumol. 2018;54:639–640.