Bronchiolitis obliterans (BO) after hematopoietic stem cell transplantation (HSCT) is a serious, potentially fatal complication, which appears in association with chronic graft-vs-host disease (GVHD).1 Lung function testing, and to a lesser but growing extent, computed tomography (CT) are the most important diagnostic tests in the detection of post-HSCT BO.2 Systemic corticosteroids remain the cornerstone of treatment, but one of the most important new therapies is ruxolitinib, a drug that is showing encouraging results in patients with GVHD.3
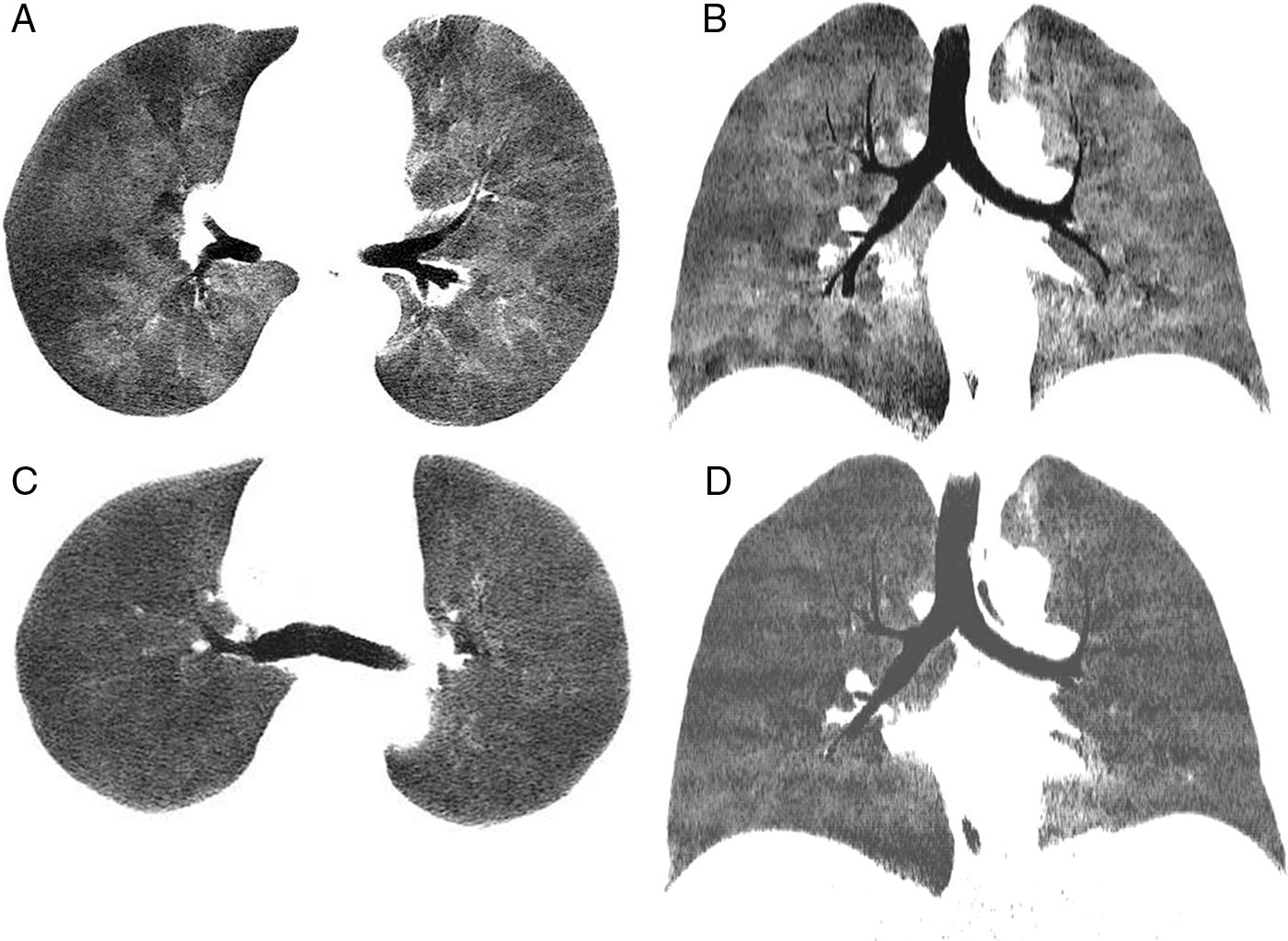
We report the case of a 39-year-old man with a history of Stage IV diffuse large B-cell lymphoma treated with several lines of chemotherapy who, after achieving complete remission, underwent HSCT from a matched donor in April 2015. Ten months after the procedure, the patient developed GVHD with cutaneous, gastrointestinal and pulmonary manifestations that did not respond favorably to treatment with corticosteroids and extracorporeal photopheresis. Lung function tests prior to developing GVHD were normal, but subsequently showed an obstructive pattern of moderate intensity, with forced expiratory volume in 1 second (FEV1) 59% predicted value, forced vital capacity (FVC) 78%, and FEV1/FVC 68%, along with a 71% decrease in CO diffusion capacity from pre-GVHD values. Dynamic computed tomography (dCT) of the chest in inspiration and expiration showed extensive areas of air trapping in both lungs (Fig. 1A and B), while infectious complications were ruled out. Bronchoalveolar lavage revealed no opportunistic infections. Given these findings, a diagnosis of BO refractory to corticosteroids and extracorporeal photopheresis associated with post-HSCT GVHD was given. The patient received ruxolitinib and achieved progressive improvement of the GVHD manifestations, including BO. Four months after starting ruxolitinib, lung function tests showed significant improvement, with an increase in FEV1 (72%), FVC (80%), and FEV1/FVC (71%), although mild air trapping persisted on plethysmography, with a residual volume of 128% and residual volume/total lung capacity ratio of 127%. Reduced signs of air trapping in the expiratory phase were also observed on dCT 3 months after starting ruxolitinib (Fig. 1C and D).
Chest minIP (Minimum Intensity Projection) CT axial (A) and coronal (B) images in expiration showing a marked mosaic attenuation pattern in the pulmonary parenchyma, with geographical regions of low density alternating with areas of greater attenuation. The areas of lower density correspond to air trapping. Chest minIP (Minimum Intensity Projection) CT axial (C) and coronal (D) images in expiration showing less heterogeneity and greater uniformity of the attenuation of the pulmonary parenchyma with compared to images (A) and (B).
BO is the most common non-infectious pulmonary complication of HSCT (and among the most serious), and one of the most important risk factors is the presence of chronic GVHD. Clinical presentation of BO is often insidious, and symptoms are non-specific (cough, dyspnea), although 20% of patients can be asymptomatic.4 The National Institutes of Health of the United States specify the following criteria for the diagnosis of post-HSCT BO: (1) demonstrated airflow obstruction (FEV1/FVC <0.7 and FEV1 <80% of predicted value); (2) air trapping on CT, residual volume >120% predicted or histological confirmation of BO; and (3) absence of respiratory tract infection.5 The most common CT findings are: air trapping, thickening of the bronchial walls, mosaic attenuation pattern, and bronchial dilation.6 The long-term prognosis of BO is generally poor (5-year survival ranges between 13% and 56%),4 and the aim of treatment is to prevent progression of airflow obstruction. Treatment with systemic corticosteroids continues to be the mainstay of BO treatment, although other therapeutic options have been used in combination with extracorporeal photopheresis, corticosteroids and/or inhaled bronchodilators, montelukast, ofatumumab, and bortezomib.7 Ruxolitinib is a new targeted therapy that selectively inhibits Janus kinases which interfere in the synthesis of various cytokines and growth factors required for hematopoiesis and immune function; its efficacy in the treatment of corticosteroid-resistant GVHD has recently been demonstrated.8,9 In our case, the clinical benefit of ruxolitinib was noted in the first weeks after administration, with corresponding clinical, spirometric, and radiological improvements. Very few descriptions are available in the literature of cases of post-HSCT BO responding to treatment with ruxolitinib, and we believe that the case presented illustrates the benefit of this promising drug in patients with post-HSCT BO, while at the same time reminding us of the importance of dCT studies to correlate radiological findings with spirometry in these patients.10
Please cite this article as: Gorospe Sarasúa L, Chinea-Rodríguez A, Almonacid-Sánchez C, Almeida-Aróstegui NA. Mejoría de la bronquiolitis constrictiva tras trasplante de progenitores hematopoyéticos: demostración radiológica en paciente tratado con ruxolitinib. Arch Bronconeumol. 2018;54:640–642.