Muscle dysfunction is one of the most extensively studied manifestations of COPD. Metabolic changes in muscle are difficult to study in vivo, due to the lack of non-invasive techniques. Our aim was to evaluate metabolic activity simultaneously in various muscle groups in COPD patients.
MethodsThirty-nine COPD patients and 21 controls with normal lung function, due to undergo computed axial and positron emission tomography for staging of localized lung lesions were included. After administration of 18-fluordeoxyglucose, images of 2 respiratory muscles (costal and crural diaphragm, and rectus abdominus) and 2 peripheral muscles (brachial biceps and quadriceps) were obtained, using the standard uptake value as the glucose metabolism index.
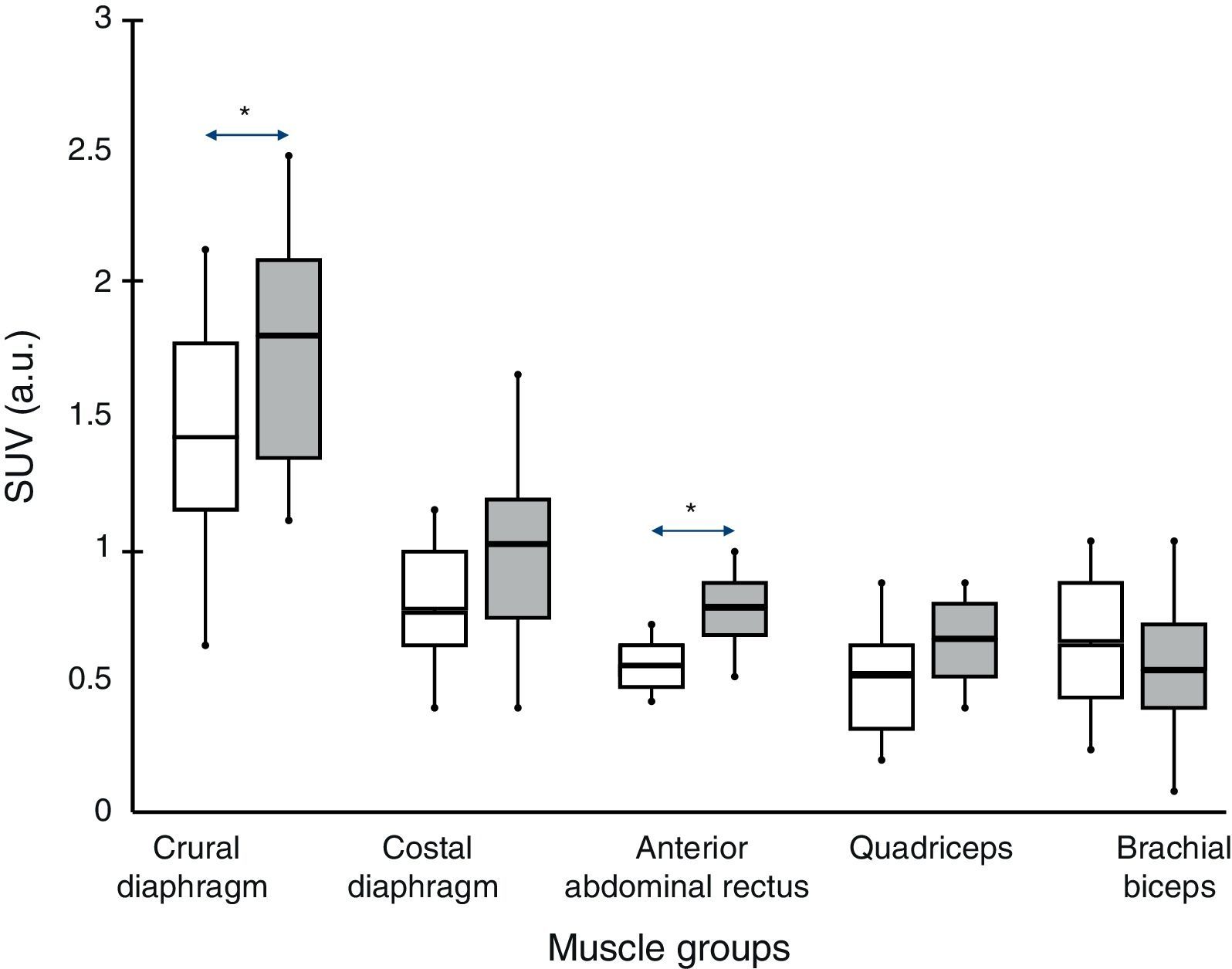
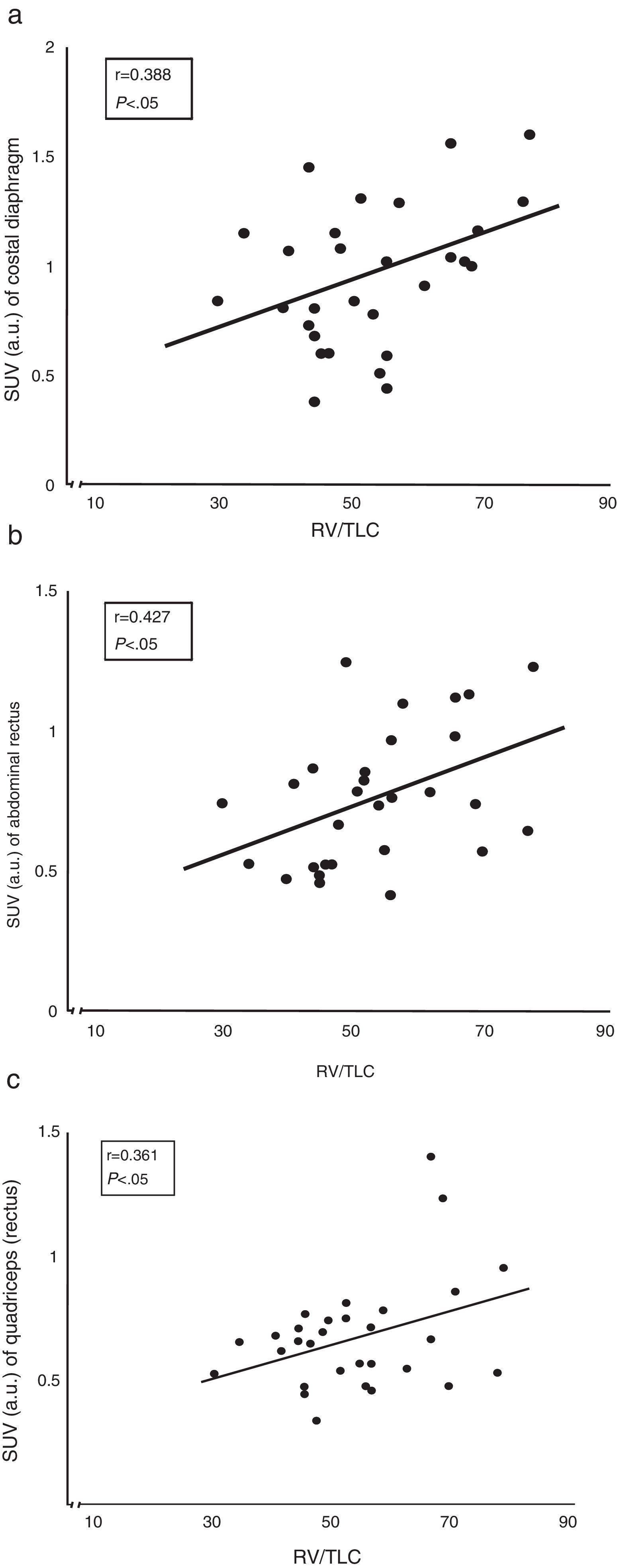
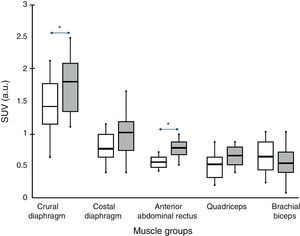
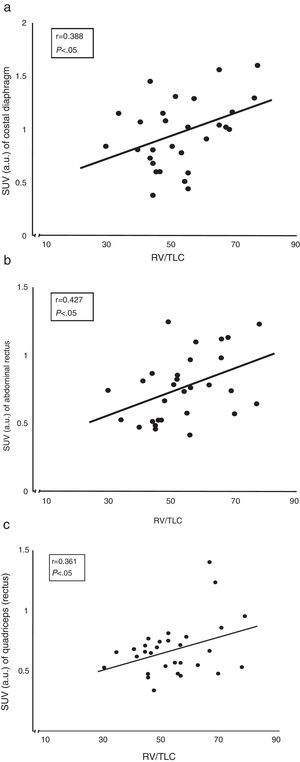
ResultsStandard uptake value was higher in both portions of the diaphragm than in the other muscles of all subjects. Moreover, the crural diaphragm and rectus abdominus showed greater activity in COPD patients than in the controls (1.8±0.7 vs 1.4±0.8; and 0.78±0.2 vs 0.58±0.1; respectively, P<.05). A similar trend was observed with the quadriceps. In COPD patients, uptake in the two respiratory muscles and the quadriceps correlated directly with air trapping (r=0.388, 0.427 and 0.361, respectively, P<.05).
ConclusionsThere is greater glucose uptake and metabolism in the human diaphragm compared to other muscles when the subject is at rest. Increased glucose metabolism in the respiratory muscles (with a similar trend in their quadriceps) of COPD patients is confirmed quantitatively, and is directly related to the mechanical loads confronted.
La disfunción muscular es una de las manifestaciones sistémicas más estudiadas en la EPOC. Las alteraciones metabólicas musculares son difíciles de estudiar in vivo, debido a la falta de técnicas no invasivas. El objetivo fue evaluar sincrónicamente la actividad metabólica de diferentes grupos musculares en pacientes con EPOC.
MétodosSe incluyeron 39 pacientes y 21 controles (función pulmonar normal), candidatos a realización de tomografía axial computarizada y por emisión de positrones para estadificación de lesión pulmonar localizada. Tras infusión de 18-fluor-deoxi-glucosa, se captaron imágenes de 2 músculos respiratorios (porciones costal y crural del diafragma, y recto abdominal) y 2 músculos periféricos (cuádriceps y bíceps braquial), utilizando como índice de metabolismo glucídico el standard uptake value.
ResultadosEste índice fue superior en ambas porciones del diafragma comparado con el resto de los músculos en todos los sujetos. Además, el diafragma crural y el recto del abdomen mostraban mayor actividad en los pacientes con EPOC que en los controles (1,8±0,7 vs. 1,4±0,8; y 0,78±0,2 vs. 0,58±0,1; respectivamente; p<0,05). El cuádriceps mostraba una tendencia similar. En los pacientes con EPOC los niveles de captación de ambos músculos respiratorios y del cuádriceps se correlacionaron directamente con el atrapamiento aéreo (r=0,388; 0,427 y 0,361, respectivamente; p<0,05).
ConclusionesExiste mayor nivel de captación-utilización de glucosa en el diafragma humano respecto de otros músculos en respiración tranquila. Se confirma cuantitativamente que los pacientes con EPOC tienen incrementado el metabolismo glucídico de sus músculos respiratorios (con tendencia similar para el cuádriceps), en relación directa con las cargas mecánicas que afrontan.
Chronic obstructive pulmonary disease (COPD) is a highly prevalent process, characterized by lung involvement and by the presence of numerous systemic manifestations and/or extrapulmonary comorbidities.1 These include nutritional disturbances and skeletal muscle dysfunction,2,3 which are in turn interelated and determine per se worse prognosis.4,5 Dysfunction of the respiratory muscles leads to difficulties in ventilation, especially when requirements are increased. Meanwhile, limb muscle dysfunction impairs both exercise capacity and numerous daily-life activities.6 Although some causal factors are common to the various muscle groups of the body, others are more specific.3 This is the case of changes in ventilatory mechanics that mainly affect the respiratory muscles, and the loss of condition derived from less physical activity, that mainly affects the lower-limb muscles.3 In addition, the cellular and molecular mechanisms that contribute to dysfunction and associated structural and metabolic findings can be either general or specific to a muscle group.7 Other consequences of the above factors and mechanisms include changes in muscle bioenergetics, which becomes less efficient, at least in the case of lower-limb muscles.8 However, metabolic studies are associated with a number of important problems. On the one hand, in vivo studies require aggressive instrumentalization of the patient (e.g. catheterization of vessels entering and leaving the muscle),8 and is not always technically possible. On the other hand, in vitro studies require the immediately processing of biopsy samples.9 Therefore, few studies have evaluated the metabolic changes occurring in muscles with difficult access, such as breathing muscles, and most of these are performed using invasive procedures such as biopsy.10–12 However, greater understanding of the use of energy substrates in the muscles of COPD patients may be useful in the management of muscle dysfunction and changes in body composition and nutritional status often associated with the disease.
Imaging techniques have advanced dramatically in recent years. One such technique, positron emission tomography (PET), provides guidance on the origin of various injuries based on the rate of uptake and utilization of key metabolites. This technique was used in 2005 to assess the activity of thoracic and abdominal muscles of COPD patients, and more qualitative activity was observed among healthy subjects.13 A few years later, Osman et al. (2011) confirmed these findings by combining PET with computed tomography (CT) in inspiratory muscles.14 We hypothesized that this combined technique might be useful for in vivo assessment with low invasiveness of simultaneous metabolic activity of respiratory and peripheral muscles, since structural and metabolic findings in biopsies of COPD patients are largely divergent.3,7 Current radiotracer uptake measurement techniques will also allow more accurate observations. The aim of this study, therefore, was to obtain a general but quantitative evaluation of the synchronous metabolic activity of various muscle groups in COPD patients compared with that of patients with normal lung function.
MethodsPopulationCandidates were initially all consecutive patients treated for pulmonary lesions, initially considered localized, in the Functional Unit of Lung Cancer in our institution (n=104). All subjects were scheduled for CT and PET for final disease staging. The study period was 10 months. Exclusion criteria were prior history of neoplastic disease, and other respiratory or inflammatory diseases. Patients suffering from other diseases that could cause false positives on PET, and those whose lung function tests did not strictly conform to normal or those with obstructive ventilatory impairment were also excluded. Patients in whom PET revealed extension of disease were also excluded. Sixty subjects were finally included, of whom 39 had a clinical history and pulmonary function tests consistent with the COPD.1 The rest had no respiratory history and their forced spirometry and CO diffusing capacity values were within normal limits.
Design and EthicsThis is a retrospective cross-sectional study designed in accordance with local, national and international standards of human research (including the Code of Good Scientific Practice of our institution and the Declaration of Helsinki) and the Spanish Personal Data Protection Act (Act 15/1999 of 13th December on the Protection of Personal Data [LOPD]), and approved by the institution's Ethics Committee. All subjects gave written consent to use their medical information after being informed about the objectives and possible consequences of the study.
TechniquesSymptomatology, Anthropometrics and Lung FunctionPersonal data, symptomatology and various clinical aspects, together with anthropometric measurements (weight, height, and body mass index) were collected from each patient. Respiratory function was then tested, including spirometry with bronchodilator (Datospir 92, SIBEL, Barcelona, Spain), static lung volume and airway strength was tested using body plethysmography (Masterlab, Jaeger, Würzburg, Germany), carbon monoxide diffusing capacity was determined using the inbuilt gas meter of the same equipment (“one breath” technique), and arterial blood gas (Rapidlab 860 gas analyzer, Bayer, Chiron Diagnostics, GmbH, Tuttlingen, Germany). SEPAR recommendations were followed in all cases, except in gases, for which the published reference values for the local population were used.15–17
Positron Emission Tomography Combined With Computed TomographyAfter fasting for 4h, baseline capillary blood glucose (Accu-Chek Aviva, Roche Diagnostics GmbH, Mannheim, Germany) was determined. If the glucose level was above 140mg/dl, a rapid insulin dose was administered according to the standardized protocol, following which tests were performed every 15min until values fell to below 140mg/dl. Once this level was achieved, all patients were administered the radiotracer 18-fluoro-deoxy-glucose (18F-FDG) at a dose of 0.1mCi/kg. Whole-body PET/CT images were obtained after waiting for a minimum of 45min, and the carbohydrate metabolic activity was quantified in different muscle groups using the standard uptake value.18,19 The variable obtained by this method is the ratio between the level of radioactivity at a particular location and the level of radioactivity that would be found in the hypothetical case of random distribution throughout the body, expressed in arbitrary units. The muscles studied were diaphragm, representing predominantly inspiratory muscles; the rectus abdominis (umbilical region and iliac crest), as the accessory expiratory muscle; the quadriceps (anterior portion of the rectum, together with the greater trochanter) and the biceps brachii (middle third of the arm). In the two limb muscles, the value obtained in the non-dominant side of the body was taken. In the case of the diaphragm, different images and quantification were obtained for the costal portion (apposition area) and crural portion (at the level of the 12th dorsal vertebral body).
Statistical AnalysisQuantitative variables with normal distribution are presented as mean value±standard deviation, while those without such distribution are shown as median±interquartile range. Non-normal distribution variables in different muscles in the same subject were compared using the Wilcoxon test, whereas comparisons between groups were performed using the non-parametric Mann–Whitney U test. Finally, the relationship between quantitative variables was analyzed using the Spearman correlation coefficient. In all cases, statistical significance was defined as less than 0.05. SPSS version 14.0 (IBM, New York, NY, USA) was used in all analyses.
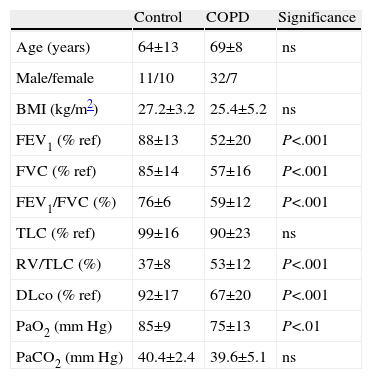
ResultsClinical CharacteristicsDemographic and clinical characteristics, including anthropometric and functional details, are shown in Table 1. The sample basically consisted of subjects aged between 60 and 70 years with good nutritional status. In the control group, 6 patients had been smokers, with an average consumption of 15 packs/year. As is implicit in the design, several functional variables were modified in COPD patients.
Personal Data, Anthropometric and Lung Function Characteristics of COPD Patients and Subjects With Normal Lung Function (Controls).
| Control | COPD | Significance | |
| Age (years) | 64±13 | 69±8 | ns |
| Male/female | 11/10 | 32/7 | |
| BMI (kg/m2) | 27.2±3.2 | 25.4±5.2 | ns |
| FEV1 (% ref) | 88±13 | 52±20 | P<.001 |
| FVC (% ref) | 85±14 | 57±16 | P<.001 |
| FEV1/FVC (%) | 76±6 | 59±12 | P<.001 |
| TLC (% ref) | 99±16 | 90±23 | ns |
| RV/TLC (%) | 37±8 | 53±12 | P<.001 |
| DLco (% ref) | 92±17 | 67±20 | P<.001 |
| PaO2 (mm Hg) | 85±9 | 75±13 | P<.01 |
| PaCO2 (mm Hg) | 40.4±2.4 | 39.6±5.1 | ns |
DLco: carbon monoxide diffusing capacity, FEV1: forced expiratory volume in one second, FVC: forced vital capacity, BMI: body mass index, ns: not significant; PaCO2: partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood; RV: residual volume, TLC: total lung capacity.
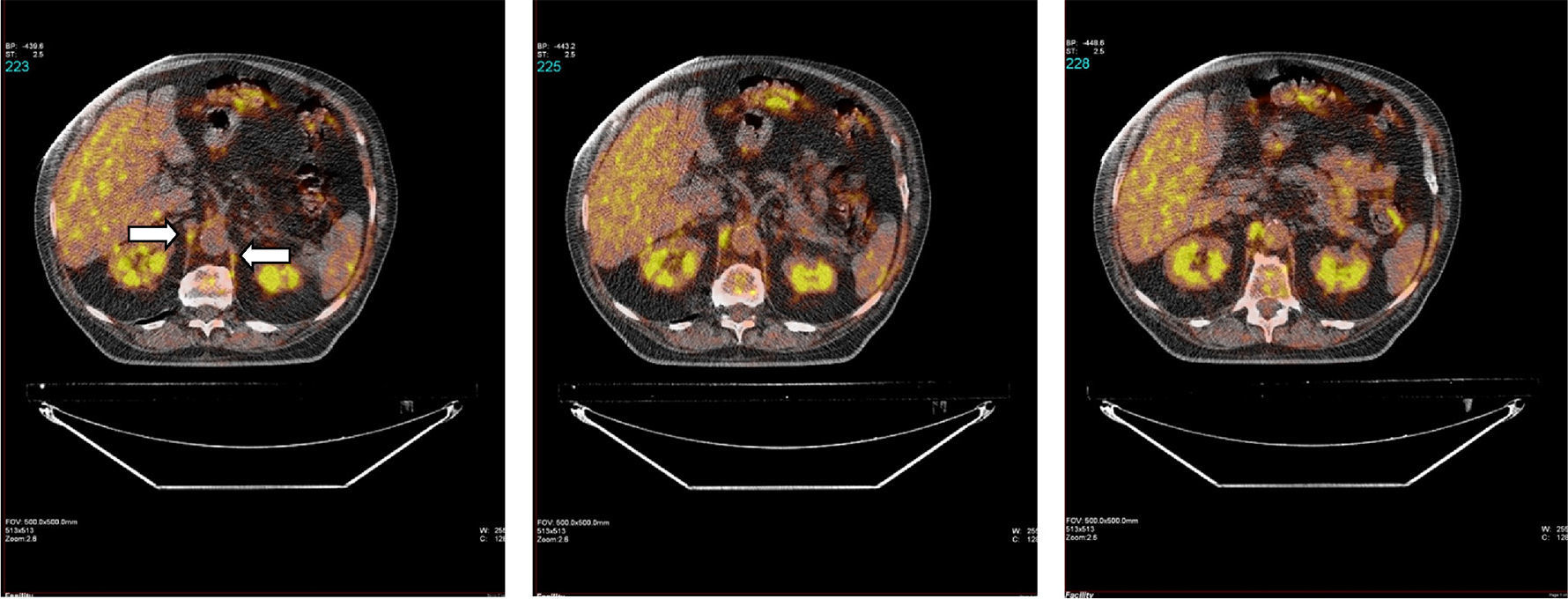
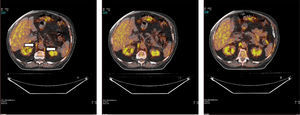
Fig. 1 shows a typical example of 18F-FDG radiotracer uptake by the crural diaphragm in COPD patients. Uptake by different muscle groups for the two study groups is shown in Fig. 2.
In all subjects, both in COPD patients and individuals with normal lung function, increased radiotracer uptake by the diaphragm was observed (crural and costal portions) compared to the rest of the muscles (rectus abdominis, quadriceps and biceps brachii).
Comparison Between GroupsIn COPD patients, levels higher than control subjects were observed in the two portions of the diaphragm (although the difference was only significant for the crural portion, 1.8±0.7 vs 1.4±08 respectively; P<.05) and rectus abdominis (0.78±0.2 vs 0.58±0.1 respectively, P<.05). The portion of the quadriceps anterior rectus also showed a similar trend, although without reaching statistical significance.
Correlation Between Radiotracer Uptake and Lung FunctionThe correlation between air trapping (represented by the residual volume vs total lung capacity ratio) and the extent of uptake of both the costal diaphragm and the rectus abdominis and quadriceps of COPD patients was the most interesting (Fig. 3).
DiscussionThe most relevant findings of this study are: (1) The combined use of CT-PET with 18F-FDG can be useful to simultaneously assess the glucose metabolism of different muscle groups (respiratory and peripheral) in vivo and in COPD patients; (2) at rest and under calm breathing conditions, heterogeneous uptake in the different muscle groups studied was observed, being higher in the main inspiratory muscle, the diaphragm, both in healthy subjects and COPD patients; (3) under the foregoing circumstance metabolic activity of the respiratory muscles of COPD patients is known to be higher than that of control subjects, and this was quantitatively confirmed, with a similar trend being observed the quadriceps; and (4) in COPD patients there is a direct relationship between the degree of air trapping and metabolic activity of both respiratory muscles and quadriceps.
The results obtained confirm that the metabolic activity at rest is different in different muscle groups, logically being higher in the respiratory muscles, since they contract regularly. In this regard, oxygen consumption of these muscles at rest is known to represent about 2%–5% of the whole organism in healthy subjects, but increases to more than double in well-nourished COPD patients.20,21 The use of oxygen in the aerobic pathways involves a proportional degradation of metabolic substrates such as glucose to obtain energy. It is therefore interesting to confirm that metabolic activity in the respiratory muscles of COPD patients is higher than that of healthy subjects, and a similar trend is observed in the peripheral muscles. Furthermore, the level of muscle metabolism is proportional to variables representative of changes in the respiratory drive. All this is broadly consistent with previous studies using other techniques.8,20,21 On the one hand, we know that mechanical system loads increase the work of breathing, and this leads to increased oxygen and energy consumption. Calculation of the former corresponds to approximately 1–2ml per extra ventilation liter in healthy subjects, and to 3–6ml/l in COPD patients.20,21 Moreover, several studies have shown that the structural and metabolic changes that occur in the muscles of COPD patients vary according to contractile groups; they all show functional impairment, but to a different degree.22 Furthermore, the respiratory muscles combine greater structural injury with increased presence of inflammatory cells and cytokines, oxidative stress, and apoptosis signs23,24; but they also exhibit more favorable changes such as increased capillarity, key aerobic pathway enzyme activity, and percentage of predominantly oxidative fibers.12,25–27 A novel and intriguing finding of this study is the higher radiotracer uptake observed in the crural diaphragm, which has a more anchoring function, compared to the costal portion, which acts mainly as a plunger. No previous studies have measured the differential energy consumption between different portions of the diaphragm, although this might be related to the known heterogeneity of its fiber composition, the regional glycogen content, or the different oxidative capacity of fast fibers.28,29
Regarding the two previous studies that used PET in the assessment of metabolic muscle activity,13,14 one of the notable developments of this study is the use of a radiotracer uptake quantification technique that allowed greater accuracy. Obviously, a purely qualitative assessment may show major differences between different muscles or individuals, but minor variations cannot be statistically assessed. Another innovation is the distinction between the activities of the costal and crural portions of the diaphragm, whose functions are different (plunger and anchorage of surrounding structures, respectively). Moreover, this study simultaneously assessed limb muscles, in which biopsies from previous studies have shown substantially different findings to those of the respiratory muscles. The simultaneous evaluation of peripheral and respiratory muscles is also interesting, because under certain circumstances both contractile groups seem to compete for blood flow and the consequent supply of metabolic substrates.30
In this study, the quadriceps muscle showed a trend toward increased glucose uptake in COPD patients. This had already been suggested in a previous study showing that this muscle has increased energy consumption in COPD patients on any activity level,8 despite a decreased concentration of aerobic structural elements, accompanied by oxidative stress, signs of lesion and apoptosis.24,31–37 This change in metabolic efficiency (bioenergetics) is not reversed with training, which suggests involvement of factors unrelated to deconditioning.
The upper-limb muscles, meanwhile, show an opposite trend to that observed in the quadriceps. This might be explained by the few structural and metabolic changes caused by COPD in these muscles.9,38 Generally speaking, it could be argued that the known heterogeneity of biopsy findings from different muscle groups is also reflected in the diversity in glucose uptake evidenced in the present study. This shows the diversity of disease impact, which is more extreme in the case of the respiratory muscles. Moreover, the increase in glucose-linked metabolic activity follows a dose–response pattern relative to the mechanical load (represented here by air trapping) sustained by patients. The relationship between increased static lung volume and the metabolic activity of inspiratory muscle (diaphragm in this case) probably depends on both changes in the breathing pattern and the gradual emergence of positive end-expiratory pressure, factors that must be overcome by muscle contraction before generating airflow. This would entail greater demand, and thus higher energy consumption. The expiratory muscle (rectus abdominis) is known to be part of a contractile group acting in COPD patients even at rest, since it is needed to overcome airway expiratory resistance. This activity is further increased by exercise, both to increase expiratory airflow and prevent hyperinsuflation.39 In subjects with normal spirometry, by contrast, glucose uptake was similar to that seen in limb muscles. This is logical, given the inactivity of the latter during the scan and the assumed relative passivity of the former during breathing at rest.40 On the contrary, abdominal muscles are also known to be actively recruited in healthy subjects during exercise (even light), with functional results similar to those mentioned in COPD patients.39
This study was limited to quiet breathing and the burdens of the disease in stable phase. The distribution of metabolic muscle activity and how this changes in COPD patients subject to different levels of respiratory load or general physical activity should also be evaluated. In these situations, several factors may influence metabolic capacity and activity, such as capillarity and blood flow, hemoglobin levels, diffusion of gas in tissue, and the use of oxygen and metabolic substrates, among others. Moreover, some studies show how these variables may behave differently in different muscle groups since, for example, blood flow is known to be reserved to the quadriceps during general exercise at high loads, but perfusion of the respiratory muscles of the thoracic box is restricted.30 Another interesting situation to be explored in the future is exacerbation of the disease. Undoubtedly, both the increased mechanical load and the systemic and pulmonary inflammation accompanying these situations could modify the metabolic activity of various muscle groups.
Potential clinical implications. The direct and linear relationship between mechanical load and muscle energy consumption observed in COPD patients in this study may have implications for patient management. Thus, patients with higher degree of air trapping are probably more susceptible to respiratory muscle failure in ventilatory stress situations. However, this hypothesis, which would involve stepping up monitoring of this subpopulation during severe exacerbations, should be confirmed by future studies involving instrumentally increased ventilatory loads.
Combined use of PET/CT, meanwhile, could become useful in identifying patients at risk of or susceptible to therapeutic interventions (e.g. selective muscle training) due to more or less altered bioenergetics in different muscles, without the need for invasive and technically complex procedures (biopsy, arterial and venous catheterization for measurement of local oxygen consumption). This metabolic characterization could be performed in a clinical setting. Of course, the low invasiveness and ease of use of PET/CT may also pave the way for research into muscle metabolism in respiratory diseases, facilitating understanding of the changes associated with these conditions.
Limitations and SpecificitiesA potential limitation of this study was the inclusion of patients with mostly localized pulmonary neoplasia, making it impossible to absolutely rule out interference from this pathology. However, the patients studied were suffering from a non-disseminated disease located far from the areas of interest of the study. Furthermore, a small percentage of patients were using drugs that may occasionally trigger effects on the muscle structure and/or function. Drugs of this type used in the study were corticosteroids (5% of the patients had received them some months previously, in previous exacerbations), calcium antagonists (6%) and insulin (5%).
The second limitation is related to the chosen expiratory muscle. The anterior rectus is probably the abdominal muscle less involved in the generation of positive pressure in the cavity. However, it is the most accessible to the technique used, and the results show the advantages of this choice (differences between controls and COPD patients, and relationship to functional impairment in the latter).
A third feature of this study that may be considered a potential limitation is derived from the need to choose a specific radioisotope. The 18F-FDG isotope is routinely used to stage patients with suspected cancer, and provides reliable information of the use of glucose in tissue, and thus the general level of metabolic activity. Glucose use is known to mark the start of usual energy obtention pathways, both anaerobic and aerobic, and is therefore a very sensitive element for assessing overall metabolism. However, it is not very specific. Other complementary tracers may be useful in studies designed to explore metabolic pathways or more specific pathway points.
Finally, the scanned area may have partially included some additional muscle in some cases, due to overlapping planes (e.g. in the abdomen). These muscles would in any event be local, and for classification purposes would form part of a contractile group considered functionally homogeneous.
This study demonstrates that PET/CT using 18F-FDG radiotracer is able to quantify the simultaneous metabolic activity of various muscle compartments in humans. This activity is heterogeneous, and is greater in respiratory muscles than those located in the limbs. As previously described (but not quantified), the respiratory muscles of COPD patients at rest show increased metabolic activity compared to those of subjects with normal lung function, but our study also shows a similar trend in lower-limb muscles. Muscle activity in patients is also proportional to the degree of lung function impairment, suggesting a cause-effect relationship, with potential clinical implications.
FundingFunded by: SAF2007-62719, SAF2011-26908, 2009SGR393 and CIBERES.
Conflicts of InterestThe authors declare no conflict of interest.
We would like to thank radiologists Iván Vollmer and Ángel Gayete for their help in identifying anatomical structures, and to nurses Ángela Roig and Laura Muñoz for their help in performing lung function tests.
Please cite this article as: Sancho-Muñoz A, Trampal C, Pascual S, Martínez-Llorens J, Chalela R, Gea J, et al. Utilización de glucosa en los músculos de pacientes con enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2014;50:221–227.