Non-cystic fibrosis bronchiectasis (bronchiectasis) is a heterogenous chronic pulmonary condition that has been linked to heightened healthcare utilization, reduced quality of life, and impaired lung function. Notably, these patients face an increased risk of mortality, as evidenced in a large study conducted by Sin and colleagues. The study revealed an increased rate of all-cause mortality in patients with bronchiectasis compared to a control group (1609 vs. 134 per 100,000 person-years).1 The establishment of large multinational and multicenter bronchiectasis registries, along with other studies, has contributed to an enhanced understanding of mortality risks and other outcomes in patients with bronchiectasis.2,3
Exacerbation of bronchiectasis stands out as one of the risk factors for mortality identified in affected patients. These events, characterized by acute worsening of respiratory symptoms requiring a change in therapy, have been independently associated with future risk of mortality.4 Particularly, patients experiencing frequent exacerbations face a significantly higher risk.5 Therefore, research has focused on unraveling reasons behind the variability in exacerbation frequency. The colonization or infection with Pseudomonas aeruginosa has been associated with increased rates of exacerbations, hospitalizations, and mortality.6,7 Recognizing the significance of this pathogen, guidelines now recommend for the surveillance and eradication of P. aeruginosa.8,9 Additionally, non-tuberculous mycobacteria infection has been correlated with a twofold increase in the adjusted all-cause mortality in patients with lung disease.10 Patients with coexisting asthma or COPD are also at increased risk for future exacerbations and mortality, making them a distinct population of interest.11,12 Furthermore, patients with bronchiectasis encounter an increased risk of cardiovascular events and death related to lung cancer.1,13 While these clinical and microbiological characteristics offer insight into identifying patients at risk for future exacerbations and mortality, there is a need for a more nuanced understanding of factors associated with higher risk.
To address this need, multidimensional scores that evaluate these parameters have been developed and validated. The FACED score, which includes forced expiratory volume in one second, age, chronic colonization by P. aeruginosa, radiological extension, and dyspnea, has demonstrated its ability to predict both all-cause and respiratory mortality.14 Similarly, the bronchiectasis severity index or BSI, utilizing additional information such as body mass index, history of exacerbations, and hospitalizations, has proven effective in predicting future exacerbations and mortality.15 While these tools are valuable in identifying at-risk patients, they are most applicable when the disease is already established and may not be as effective in the earlier stages. Therefore, there remains a need for additional diagnostic tools to pinpoint at-risk patients.
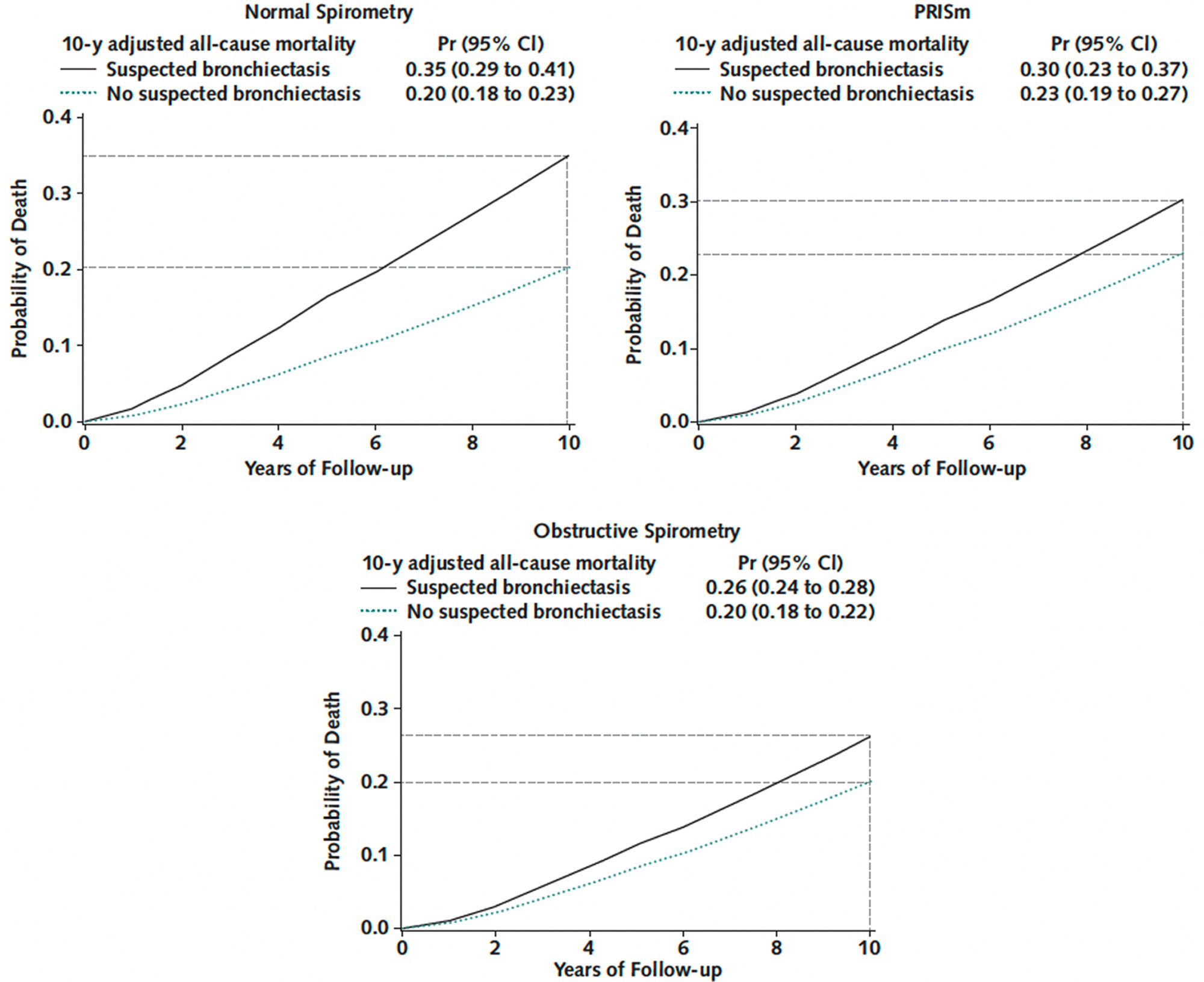
Computed tomography (CT) is a useful tool the diagnosis and classification of bronchiectasis. Nevertheless, the evaluation of airway abnormalities and other structural features in clinical practice is often subjective, and few studies have objectively quantified the extent of bronchiectasis and its association with important outcomes. Visual inspection and scoring of CT abnormalities can be variable and time-consuming, with a lack of validation studies.16,17 To overcome these limitations, automated protocols have been developed and are currently under investigation. Emerging data indicate that even subtle abnormalities on CT may be associated with poor outcomes. A study in the COPDGene cohort focused on unique subgroups of patients who smoke with incidental bronchiectasis on CT: those with normal spirometry and those with preserved ratio and impaired spirometry (PRISm).18 An artificial intelligence (AI)-based measuring tool was employed to quantify air-way-to-artery ratios (AAR) throughout the entire bronchial Tree.18,19 Then, the percentage of airways with AAR >1 was calculated, with a value higher than 1% consistent with bronchiectasis. In this study, the primary outcome of “suspected bronchiectasis” was defined as an AAR >1 greater than 1% plus 2 of the following: cough, phlegm, dyspnea, and history of two or more exacerbations. Participants with normal and obstructive spirometry meeting the criteria for suspected bronchiectasis had an increased risk for all-cause mortality, compared with the no-suspected-bronchiectasis group (Fig. 1). In participants with PRISm, the increase in mortality risk was inconclusive. In addition, the subgroup of smokers with normal spirometry and suspected bronchiectasis had a 15% higher risk for all-cause mortality.
Mortality curve plots by suspected bronchiectasis status across spirometry-based groups. Left upper. Participants with a history of smoking and normal spirometry (defined as FEV1/FVC ratio ≥0.7 and FEV1% predicted ≥80). Right upper. Participants with a history of smoking and preserved ratio impaired spirometry, PRISm (defined as FEV1:FVC ≥0.7 and FEV1% predicted <80). Lower. Participants with a history of smoking and obstructive spirometry (defined as FEV1/FVC <0.7). Data are from a Cox survival model adjusted for age, sex, race, body mass index, smoking status, pack-years smoked, the number of comorbidities, FEV1 after bronchodilator use, oxygen saturation, CT measures of emphysema and airway wall thickness, and CT scanner make/model. Suspected bronchiectasis was defined as more than 1% of bronchi with an airway-to-artery ratio >1 on chest computed tomography scans plus two or more of the following: cough, phlegm, dyspnea, and history of two or more exacerbations. Participants meeting criteria for suspected bronchiectasis (solid black lines) have an increased risk for all-cause mortality compared with those without suspected bronchiectasis (dotted green lines) across the three spirometric groups. Horizontal broken lines mark estimated probabilities of 10-year mortality. Vertical broken lines mark the 10-year follow-up. Pr: probability; CI: confidence interval; PRISM: preserved ratio impaired spirometry; CT: computed tomography; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity.
What are the implications of these findings? Traditionally, bronchiectasis is typically diagnosed in later stages of the disease. This is the result of the unspecific nature of respiratory symptoms in the earlier stages and the emergence of significant clinical symptoms only when advanced disease is established or if there is a history of recurrent exacerbations. Automated protocols using AI, has the potential to more accurately analyze parenchymal, airway, and vessel abnormalities, potentially detecting at-risk populations at earlier stages.18,19 While not yet integrated into clinical practice, the referenced study offers an example of AI's potential as a predictive tool in bronchiectasis for at-risk individuals. Despite this promising glimpse, further studies are required to validate and replicate these findings, and it will take time before these diagnostic tools can become widely available. The integration of AI as a diagnostic tool in bronchiectasis could herald the beginning a new era of phenotyping. Prompt diagnosis and classification of these patients may improve referral patterns and facilitate earlier interventions, such as airway clearance and anti-inflammatory therapies. Such early interventions hold the potential to decrease exacerbations, impede disease progression, and ultimately reduce the risk of mortality.
FundingAAD is funded by the United States National Heart Lung Blood Institute, R01-HL149861, R01-HL164824.
Conflict of InterestsDJM reported personal fees from AstraZeneca, GSK, Amgen, Sanofi/Regeneron. AAD reported personal fees from Boehringer Ingelheim and having a patent for Methods and Compositions Relating to Airway Dysfunction pending (701586-190200USPT).