The Pickwick project is a prospective, randomized and controlled study, which addresses the issue of obesity hypoventilation syndrome (OHS), a growing problem in developed countries. OHS patients were divided according to apnea–hypopnea index (AHI) ≥30 and <30 determined by polysomnography. The group with AHI≥30 was randomized to intervention with lifestyle changes, noninvasive ventilation (NIV) or continuous positive airway pressure (CPAP); the group with AHI <30 received NIV or lifestyle changes.
The aim of the study was to evaluate the efficacy of NIV treatment, CPAP and lifestyle changes (control) in the medium and long-term management of patients with OHS. The primary variables were PaCO2 and days of hospitalization, and operating variables were the percentage of dropouts for medical reasons and mortality.
Secondary medium-term objectives were: (1) to evaluate clinical–functional effectiveness on quality of life, echocardiographic and polysomnographic variables; (2) to investigate the importance of apneic events and leptin in the pathogenesis of daytime alveolar hypoventilation and change according to the different treatments; (3) to investigate whether metabolic, biochemical and vascular endothelial dysfunction disorders depend on the presence of apneas and hypopneas and (4) changes in inflammatory markers and endothelial damage according to treatment.
Secondary long-term objectives are to evaluate: (1) clinical and functional effectiveness and quality of life with NIV and CPAP; (2) changes in leptin, inflammatory markers and endothelial damage according to treatment; (3) changes in pulmonary hypertension and other echocardiographic variables, as well as blood pressure and incidence of cardiovascular events, and (4) dropout rate and mortality.
El proyecto Pickwick es un estudio prospectivo, aleatorizado, abierto y controlado con grupos en paralelo que intenta dar respuesta a los interrogantes del síndrome de hipoventilación-obesidad (SHO), una enfermedad creciente en los países desarrollados. Para ello, pacientes con SHO fueron divididos en pacientes con índice de apneas-hipoapneas (IAH) ≥30 y <30 mediante polisomnografía. El grupo con IAH ≥30 se aleatorizó a tratamiento mediante estilo de vida saludable, ventilación no invasiva (VNI) o presión en la vía aérea positiva continua (CPAP). El grupo con IAH<30, a VNI o estilo de vida saludable.
Su objetivo ha sido evaluar la eficacia del tratamiento con ventilación VNI, CPAP y estilo de vida saludable (control) a medio y largo plazo en el SHO, analizando como variable primaria la PaCO2 y los días de hospitalización, respectivamente, y como variables operativas el porcentaje de abandonos por razones médicas y mortalidad.
Los objetivos secundarios a medio plazo fueron: 1) evaluar la eficacia clínica-funcional, en calidad de vida, en variables polisomnográficas y ecocardiográficas; 2) investigar la importancia de los episodios apneicos y de la leptina en la génesis de la hipoventilación alveolar diurna y el cambio con los diferentes tratamientos; 3) investigar si las alteraciones metabólicas, bioquímicas y disfunción endotelial vascular dependen de la presencia de apneas e hipoapneas, y 4) cambio de marcadores inflamatorios y de daño endotelial con los tratamientos.
Los objetivos secundarios a largo plazo fueron: 1) evaluar la eficacia clínico-funcional y en calidad de vida con VNI y CPAP; 2) cambio de la leptina, marcadores inflamatorios y de daño endotelial con los tratamientos; 3) cambio de la hipertensión pulmonar y otras variables ecocardiográficas, así como en la hipertensión arterial e incidencia de episodios cardiovasculares, y 4) frecuencia de abandonos y mortalidad.
Obesity, dubbed the “global epidemic of the 21st century”, is a growing phenomenon in the developed world, and now affects one third of the adult population.1 One obesity-related disorder is obesity hypoventilation syndrome (OHS), characterized by chronic hypercapnic respiratory failure not secondary to other causes, episodes of sleep apnea and hypoventilation.2 Less is known about the repercussions of OHS than those of sleep apnea–hypopnea syndrome (SAHS), although pulmonary hypertension and metabolic syndrome seem to be more common. The greater degree of sleep hypoxia in OHS patients may generate a greater risk for other cardiovascular events and mortality compared with obese individuals with no respiratory failure.3 The best treatment is weight loss,4 as a return to normal weight reverses respiratory failure, pulmonary hypertension and sleep disturbances.5 However, these patients find it difficult to achieve and maintain significant weight loss. Bariatric surgery is a marginal option, but it is associated with greater mortality and is usually rejected by patients. Although continuous positive airway pressure (CPAP) corrects sleep apnea in OHS patients, it does not seem to normalize daytime PaCO2 in all cases.6 The role of apnea and hypopnea in the development of daytime hypercapnea, therefore, is not entirely clear, although it has been suggested that patients who respond to CPAP may present predominantly apneic episodes, rather than alveolar hypoventilation.6 Daytime hypercapnia in SAHS has been associated with the event:interevent duration ratio, which would mean that sleep and daytime hypercapnia result from the individual's failure to recover from the hypoventilation caused by episodes of apnea, particularly during the rapid eye movement (REM) stage of sleep.7 CPAP and non-invasive ventilation (NIV) are used extensively in the treatment of chronic OHS. The effectiveness of CPAP vs NIV has been studied in only 1 randomized trial,8 in which 36 patients were selected on the basis of a favorable response to a trial CPAP treatment session. The authors concluded that clinical and blood gas improvement at 3 months was similar for both therapies. Another randomized trial compared the effectiveness of NIV with “healthy lifestyle” counseling (i.e., sleep hygiene and diet) in patients with OHS selected on the basis of mild daytime hypercapnia.9 No randomized trials have been performed in non-selected patients to determine which of these treatments is most effective (NIV vs CPAP), or which is most effective compared to a control intervention (healthy lifestyle).
Likewise, no studies with a similar design have been undertaken to show whether the complications associated with OHS (arterial and pulmonary hypertension, cardiovascular events, hospitalization and mortality) decrease more or less with any of these treatments. A new study is needed to determine whether NIV is superior to CPAP or lifestyle changes in the medium- and long-term, and to validate the current widespread use of NIV in OHS patients.
We undertook a prospective, open-label, parallel group, randomized controlled trial in OHS patients to evaluate the medium- and long-term efficacy of NIV, CPAP and lifestyle changes (control). The primary outcome was PaCO2 and days of hospital stay, respectively. Operating variables were dropout for medical reasons (%) and mortality.
Logistical ConsiderationsThe Steering Committee of the Separ Comprehensive Research Study on Sleep (CRS on Sleep) appointed Dr. Juan Fernando Masa to head Pickwick, a project that would bring together clinical and basic research to find more comprehensive answers to the incognitos surrounding this increasingly widespread disease (see Addendum 2).
Patient selection in participating centers began in 2009 (a list of centers is given in Addendum 3).
The following precepts were established in advance: (a) monthly newsletters would be sent by the principal investigator to all co-investigators comparing the progress made by different trial centers, encouraging recruitment efforts, and reporting on the latest developments; (b) investigator meetings would be held to coincide with SEPAR conferences and the winter meeting; and (c) the authorship policy was defined, with an estimation of the number of articles to be published and the names of the authors, based on the number of patients contributed to the trial (Addendum 4).
Objectives of the StudyThe short-, medium-, and long-term primary and secondary objectives are summarized in Table 1.
Primary and Secondary Objectives.
| Primary |
| To evaluate the long-term efficacy of NIV treatment vs CPAP or a sleep hygiene-diet regimen (control arm), using all-cause days of hospital stay as the primary outcome variable |
| To evaluate the medium-term efficacy of NIV, CPAP and a sleep hygiene–diet regimen (control arm), using PaCO2 as the primary outcome variable |
| Medium-term secondary objectives |
| To determine clinical and functional effectiveness on quality of life, polysomnography and echocardiography variables |
| To study the importance of apneic episodes and leptin levels in the pathogenesis of daytime hypoventilation |
| To analyze the effect of study treatments on changes in leptin levels |
| To determine whether metabolic, biochemical and vascular endothelial dysfunctions depend on the presence of apnea–hypopnea |
| To study the effect of study treatments on changes in inflammatory and endothelial damage markers |
| Long-term secondary objectives |
| Clinical and functional effectiveness on quality of life variables over 3 years |
| Effect of study treatments on changes in leptin levels |
| Effect of study treatments on changes in inflammatory and endothelial damage markers |
| Changes in arterial and pulmonary hypertension and other echocardiography variables |
| Effect of study treatments on the incidence of arterial hypertension and cardiovascular events |
| Percentage of dropouts and deaths |
Inclusion criteria were: patients referred to pulmonary outpatient departments of participating hospitals with stabilized hypercapnic respiratory failure (pH≥7.35, with no clinical symptoms of exacerbation in the 2 preceding months or more) secondary to obesity. OHS was defined as combined obesity (body mass index [BMI]≥30) and stabilized hypercapnic respiratory failure (PaCO2≥45mmHg) not secondary to other causes.2 Patients that met all the inclusion criteria and none of the exclusion criteria were included consecutively in the study (Table 2). Patients underwent an NIV or CPAP tolerance test, and were given detailed instructions on how to operate the equipment and a description of the expected benefits of the treatment (see Addendum 5). The study was approved by the ethics committees of all 16 centers. All patients signed an informed consent form.
Inclusion and Exclusion Criteria.
| Inclusion criteria |
| 1. Age between 15 and 80 years. |
| 2. No moderate or severe chronic obstructive pulmonary disease (FEV1>70% predicted if FEV1/FVC<70). |
| 3. Daytime hypercapnia not caused by neuromuscular, chest wall or metabolic disease. |
| 4. No narcolepsy or restless legs syndrome. |
| 5. Satisfactory outcome of a ≥30-min daytime trial CPAP/NIV therapy session. |
| Exclusion criteria |
| 1. Psychologically or physically unable to complete questionnaires. |
| 2. Patients who, due to previously diagnosed chronic disease (malignancy, severe chronic pain of all causes, severe kidney failure, and any other severe, limiting chronic disease), cannot be evaluated by means of a quality of life questionnaire. |
| 3. Inability to use CPAP/NIV devices due to chronic nasal obstruction. |
| 4. Refusal to sign the informed consent form. |
Pickwick is a prospective, multicenter, open-label, randomized, parallel group controlled trial designed to compare the medium-term (primary outcome: PaCO2) and long-term (primary outcome: days of hospital stay) efficacy of NIV, CPAP or lifestyle recommendations (sleep hygiene and diet control group) in stabilized OHS patients by means of prospective follow-up of parallel cohorts.
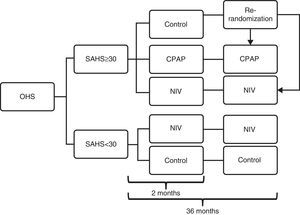
ProtocolPatients recruited for the trial underwent a standard polysomnography (PSG) (see Addendum 6) and were then randomized to 5 groups (2 randomizations, 1 in each cohort) on the basis of a computer-generated numerical list (SPSS, Chicago IL, USA), following the flow chart shown in Fig. 1: (a) patients with apnea–hypopnea index (AHI)≤30 (no or mild-moderate SAHS) and (b) patients with AHI≥30 (severe SAHS). The first cohort of patients was randomized to the NIV or control arms, and the second was randomized to the control, NIV or CPAP arms for 2 months (until the medium-term evaluation).
Study protocol flow chart. Patients initially recruited were divided into 2 cohorts: patients with apnea–hypopnea index (AHI) ≤30 (no or mild-moderate SAHS) and patients with AHI ≥30 (severe SAHS). In the ≤30 cohort, patients were randomized to the NIV or control arms, and in the ≥ 30 cohort they were randomized to the control, NIV or CPAP arms until the medium-term evaluation (2 months). Following this, patients in the control arm of the ≥30 cohort were re-randomized to the NIV or CPAP arms, and both cohorts then completed the 36 months of follow-up. CPAP: continuous positive airway pressure; NIV: non-invasive ventilation; OHS: obesity hypoventilation syndrome; SAHS: sleep apnea hypopnea syndrome.
After the 2-month evaluation, patients in the control group of the SAHS cohort were re-randomized to either the NIV or CPAP, and continued in these arms until the 36-month (long-term) evaluation. The 2 arms of the non-SAHS cohort remained unchanged.
Table 3 shows the treatment administered to each arm (control, CPAP and NIV) (see also Addendum 7).
Treatment of Study Arms Following Randomization.
| 1. Control arm: lifestyle changesThis consisted of following a 1000 calorie diet and adopting good sleeping habits (avoiding the supine position, following a regular sleep and exercise schedule, no intake of sedatives, stimulants, alcohol, tobacco or heavy meals up to 4h before bedtime). Supplementary oxygen therapy was administered if needed. In this case, the flow rate was adjusted to maintain daytime SatO2 of between 88% and 92% or PaCO2 ≥55mmHg for at least 17h/day. Baseline arterial blood gas was measured after 20min of oxygen therapy. If PaCO2 had increased ≥5mmHg or pH was <7.35, oxygen therapy was suspended (see Addendum 8). |
| 2. CPAPIn addition to lifestyle changes and oxygen therapy (if needed), home CPAP treatment was started using standard CPAP titration. |
| 3. NIVIn addition to lifestyle changes and oxygen therapy (if needed), NIV treatment was started, in the form of bi-level positive pressure with volume-assured support (see Addendum 6). |
Patients were contacted at 30 days, 2 months, and then every 3 months for a total of 2 years. After this time, they were contacted every 6 months until the end of the trial (3 years). Table 4 shows the study variables, and Table 5 shows the evaluation timeline. Compliance with CPAP/NIV or oxygen therapy was evaluated by dividing the number of hours of use (shown on the device's hour meter) by the days of treatment. All patients were contacted monthly by telephone to encourage them to adhere to the dietary regimen. Patients were considered dropouts if, following randomization, they were unwilling or unable to continue with the study. Patients were also withdrawn if they could not continue with their assigned treatment for the following medical reasons: (a) pH levels below 7.33 in a 3-monthly room air arterial blood gas test; (b) hospitalization for respiratory failure, requiring NIV for more than 5 days, or invasive ventilation for more than 3 days, or pH levels below 7.33 on room air arterial blood gas test at hospital discharge, or (c) death.
Baseline and Follow-up Study Variables.
| 1. Anthropometric and demographic data |
| 2. Morbidity and arterial pressure |
| 3. Number of hospitalisations and days of hospital stay |
| Visits to the emergency department and scheduled hospital stays in the 3 years prior to treatment and during follow-up |
| Number of admissions to the intensive care unit in the 3 years prior to treatment and during follow-up |
| 4. Number of orotracheal intubations and visits to emergency departments |
| In the 3 years prior to treatment and during follow-up |
| 5. Number of dropouts and causes |
| 6. Number of deaths and causes |
| 7. Questionnaires |
| Clinical symptoms and Epworth sleepiness scale |
| Functional Outcomes of Sleep Questionnaire (FOSQ) |
| Medical Outcome Survey – Short Form 36 (SF36) |
| Patient-perceived disease status measured on a visual analog scale |
| 8. Functional tests |
| Blood gas |
| Spirometry |
| Walk test |
| 9. Polysomnography |
| 10. Echocardiography |
| 11. Ventilator compliance by means of an hour meter |
| 12. Standard laboratory tests |
| Complete blood count, fibrinogen, renal, liver and lipid panels, C reactive protein. |
| 13. Other tests |
Timing and Type of Evaluations.
| Evaluations (12) | Symptomsa and Morbidity | In-hospital Treatment and Mortality | ABG, AP and Panels | PSG | Echo | Lab Tests |
|---|---|---|---|---|---|---|
| Baseline | x | x | x | x | x | x |
| 30 days | x | x | ||||
| 2 months | x | x | x | x | x | x |
| 6 months | x | x | ||||
| 9 months | x | x | ||||
| 12 months | x | x | x | x | x | |
| 15 months | x | x | ||||
| 18 months | x | x | ||||
| 21 months | x | x | ||||
| 24 months | x | x | x | x | x | |
| 30 months | x | x | ||||
| 36 months | x | x | x | x | x |
ABG: arterial blood gases; AP: arterial pressure; Echo: echocardiogram; PSG: polysomnography.
Sample size was calculated to detect differences in the primary outcome variables, assuming an alpha error of 0.05 and a beta error of 0.2.
Days of hospital stay. The mean hospital stay in patients receiving chronic NIV was 2.5±1.1days/year.10 We estimated that an inter-group difference of ≥0.5days/year (20% difference) with a standard deviation of ≤1.1 would be clinically relevant. For comparison with a theoretical value (2 comparisons), we estimated that a sample size of at least 40 patients would be needed in each group. Adding a loss of 25%, 54 patients would be needed per group, for a total sample size of 216 patients.
Daytime PaCO2as the primary medium-term outcome variable. Mean PaCO2 in patients treated with NIV was 45±5mmHg.11 We estimated that an inter-group difference of ≥2.5mmHg with a standard deviation of 5 would be clinically relevant. In order to compare 2 independent means (triple comparison: SAHS cohort), 64 patients would be needed per group. Adding a loss of 20%, the final number of patients per group would be 80, or 240 patients in total. For comparison with a theoretical value (2 comparisons: non-SAHS cohort), we estimated that 34 patients would be needed in each group. Adding a loss of 20% brought the final number of patients per group to 43, or 86 patients in total.
To conclude, the sample size of the SAHS cohort was determined by PaCO2 measurements, and for this purpose, a sample size of 240 patients is needed. The sample size of the non-SAHS cohort, on the other hand, was determined by duration of hospital stay, and for this purpose, a sample size of 108 patients is needed. Taken together, the 2 cohorts produce a total sample size of 348 patients.
Statistical AnalysisAll pre- and post-treatment study variables were compared intragroup, although the primary efficacy analysis was based on a comparison of pre and post-treatment inter-group differences. ANOVA was used for the preliminary analysis, followed by ANCOVA for statistically significant values (P<.05). The percentage of dropouts, deaths and their causes, adverse effects, and hours of use were analyzed. Analyses were performed in both “intention-to-treat” and “per protocol” mode. Data missing from the primary outcome variable were replaced by values obtained by multiple regression. The foregoing analyses were performed at the time of the 2-month and 3-year evaluations.
Ethical Matters- (a)
Informed consent: patients were given written information on the nature and purpose of the trial. The project was assessed and approved by the ethics committees of participating hospitals, in accordance with the declaration of Helsinki.
- (b)
During the trial, an authorized external monitoring agency has been given access to the periodic analyses of the primary outcome variable (days of hospitalization), and the number of dropouts due to medical reasons and to death. Their task is to determine whether the trial should be halted if any particular treatment was found to be significantly worse, irrespective of the trial cohort (SAHS or non-SAHS).
The trial has now reached the mid-way point. Recruitment for the SAHS group has finalized, and the intragroup (NIV, CPAP and control) efficacy analysis is currently underway in this cohort.
A total of 302 patients have been included in the trial: 221 in the SAHS group, and 81 in the non-SAHS group. In total, 49 patients have been excluded.12
To date, 70 (23%) patients have dropped out from the trial: 53 (23%) from the SAHS group, and 17 (21%) from the non-SAHS group. Three (0.01%) patients dropped out for medical reasons: 1 from the control group (prior to the second randomization) and 2 from the SAHS with NIV group. Another 7 (0.02%) patients have died: 2 from the SAHS with NIV group, 4 from the SAHS with CPAP group, and 1 from the non-SAHS control group. So far, no difference between groups in respect of dropouts for medical reasons or death has been observed.
DiscussionThis trial will conclusively show whether treatment with NIV is superior to CPAP or a sleep hygiene and diet regimen. These findings may validate the widespread, though scientifically unfounded, use of NIV in this disease. Efficacy will be evidenced through physiological and clinical variables, as well as other more specific variables such as days of hospital stay, cardiovascular events, pulmonary hypertension, or even the dropout and mortality rate. Considering that the prevalence of OHS is thought to be rising in parallel with an increase in obesity, it is of the utmost importance that the most effective treatment for this condition is defined.
The results will be released in a series of articles published at different stages of the trial:
- 1.
Stage 1, after 2 months of follow-up: (a) comparative efficacy of the 3 study treatments in terms of symptoms, respiratory function, and echocardiogram and polysomnography findings; (b) importance of apneic episodes and leptin in the pathogenesis of daytime hypoventilation; (c) association of metabolic, biochemical and vascular endothelial dysfunction disorders with apnea–hypopnea (intermittent hypoxia hypothesis) or daytime hypoventilation (sustained hypoxia hypothesis).
- 2.
Stage 2, after 3 years of follow-up: (a) comparative efficacy based on evolution of symptoms, respiratory function and complications (hospitalizations, cardiovascular events, arterial and pulmonary hypertension, dropouts and death); (b) comparative evaluation of the evolution of biological parameters associated with each treatment.
As mentioned above, daytime hypercapnia in SAHS has been associated with the event:interevent duration ratio, which would mean that sleep and daytime hypercapnia depend on the individual's failure to recover from the hypoventilation caused by episodes of apnea, particularly during the rapid eye movement (REM) stage of sleep.13 This trial will also show whether sleep apnea plays a pivotal role in the development of daytime hypercapnia.
A typical finding in patients with OHS is an increased alveolar-arterial oxygen gradient resulting from changes in respiratory mechanics caused by the build-up of fat in the chest wall and abdomen. To date, no randomized trials have evaluated the benefits of long-term oxygen therapy alone or in combination with a sleep hygiene and diet regimen in this patient population. Moreover, oxygen therapy has not yet been compared with CPAP or NIV in the context of OHS. In this trial, oxygen is prescribed at the discretion of the patient's doctor, a factor that enabled us to analyze subgroups that did or did not receive supplementary oxygen in each treatment group.
Leptin is a protein secreted by white adipose tissue. Its function is to inhibit hunger and increase energy expenditure. Obesity is thought to be associated with central resistance to leptin, leading to hyperleptinemia. Studies in animal models have shown that leptin is a powerful respiratory stimulant, and lack of this protein (or of its effect) could cause hypoventilation. Serum leptin levels normalize in SAHS patients treated with CPAP,14 although it is thought that apnea–hypopnea is the cause, rather than the result, of elevated leptin levels. Studies suggest that serum leptin levels are twice as high in OHS patients as in patients with a similar level of obesity and frequency of apnea–hypopnea episodes, but with no daytime hypercapnia.15 Leptin levels can be reduced with NIV therapy16 or weight loss. The foregoing has led researchers to hypothesize that leptin resistance could be the cause of OHS. This theory, however, has been called into question by the contradictory findings of a more recent study,17 which reported that leptin levels are lower in OHS patients with no major apnea–hypopnea than in obese patients with a similar number of obstructive episodes and no daytime hypercapnia, and that leptin levels increase with NIV. Our study will give further insight into this conundrum: we will show whether serum leptin levels decrease or increase to within normal ranges (for the level of obesity) in OHS patients in whom daytime hypercapnia has been reversed, particularly in the group with few or no episodes of apnea–hypopnea. Our findings will also show whether leptin levels in OHS patients with obstructive episodes and no reversal of hypercapnia following CPAP treatment are higher or lower than in those with reversal of hypercapnia following CPAP or NIV. In conclusion, we will show whether hyperleptinemia is the cause of hypoventilation or the result of hypoventilation or sleep apnea.
SAHS has been associated with metabolic changes and vascular endothelial dysfunction, which could be caused by either intermittent hypoxia or the increased sympathetic activity typical of these patients. Ultimately, these conditions can contribute to the development of arterial and pulmonary hypertension and cardiovascular events. Few studies have explored this phenomenon in OHS patients, but some inflammatory mediators, such as IL-6, could be more elevated in OHS patients than in SAHS patients with no daytime hypercapnia.18 Neither has the cause of this been investigated: whether OHS patients with no major apnea–hypopnea have elevated basal levels of these markers that could decrease with treatment, or whether these markers either increase or decrease due to the presence of apnea–hypopnea. Our study could provide answers to most of these questions, and will show whether inflammation at the molecular level is driven more by intermittent hypoxia (obstructive episodes) or sustained hypoxia (hypoventilation), and how this evolves with different treatments.
The dropout rate (23%) so far is below the estimated rate of 30%, but the 3-year follow-up is still ongoing. Dropout always leads to a loss of clinical trial data, as investigators collect information on days of hospital stay, type of treatment (CPAP, NIV, oxygen or lifestyle changes), and mortality after the dropout has occurred. If the dropout rate is ultimately higher in any particular treatment group (for example, higher in CPAP than in NIV), the per protocol analysis will determine whether this is related with treatment efficacy or other circumstances (for example, patient discomfort).
Risk AnalysisThe difficulties involved in conducting this study include:
- (a)
The need for a large sample size, calling for patient recruitment in several different centers.
- (b)
Three years of follow-up, with 2 pre-inclusion studies.
- (c)
Lack of information in OHS literature on the incidence of cardiovascular events per year or how these might be affected by treatment.
- (d)
The parallel cohort study design.
- (e)
Lack of information in the literature on the prevalence of severe SAHS (AHI>30) in OHS patients (see Addendum 8).
Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias, Ministry of Health and Consumer Affairs)PI050402, Sociedad Española de Neumología y Cirugía Torácica 2005 (SEPAR) and Air Liquide España.
Conflict of InterestsThe authors declare they have no conflicts of interest.
We would like to thank Vanessa Iglesias for her technical advice.
| Estefanía García-Ledesma, MD |
| Maria L. Alonso, MD, PhD |
| Teresa Gómez-García, MD |
| Ángeles Martínez, MD |
| Olga Cantalejo |
| Elena Ojeda, MD |
| Santiago J. Carrizo, MD, PhD |
| Begoña Gallego, MD, PhD |
| Odile Romero, MD |
| Mercedes Pallero, MD |
| Jesús Muñoz-Méndez, MD, PhD |
| Cristina Senent, MD |
| José N Sancho-Chust, MD |
| Erika Miranda |
| Francisco Rivas, MD |
| Laura Cancelo, MD |
The Steering Committee of the Separ Comprehensive Research Study on Sleep (CRS on Sleep) appointed Dr. Juan Fernando Masa to head Pickwick, a project that would bring together clinical and basic research to find more comprehensive answers to the incognitos surrounding the increasingly widespread incidence of OHS.
Dr. Juan Fernando Masa contacted members specializing in the field of sleep studies, non-invasive ventilation and critical care, and interested groups with at least 3 years of experience in NIV and CPAP treatment who use PSG as part of their routine practice were invited to take part in the trial. Successive versions of the protocol were discussed in 3 consecutive meetings of the CRS on Sleep, coinciding with the annual winter meeting and SEPAR meetings. Investigators remained in contact by in 2008, the final version of the protocol was approved. Organizations were approached for funding, which was obtained from SEPAR, the Fondo de Investigaciones Sanitarias (FIS) and Air Liquide. The tools needed to conduct the multicenter study were prepared, including: (1) a case report form; (2) a database hosted on a website created specifically for the purpose of the trial; (3) a procedure manual to standardize the conduct of the trial in all centers, giving a step-by-step description of the trial and copies of all the questionnaires and procedures, and (4) 3-monthly external audits to compare inter-group operating variables (dropouts due to medical causes or death). The outcome of the audits determines the continuity of the trial.
Patient selection in trial centers started in 2009 (a list of centers is given in Addendum 3).
The following precepts were established in advance: (1) monthly newsletters would be sent by the principal investigator to all co-investigators comparing the progress made in different trial centers, encouraging recruitment efforts, and reporting on the latest developments; (2) investigator meetings would be held to coincide with SEPAR conferences and the winter meeting; and (3) authorship policy was defined, with an estimation of the number of articles to be published and the names of the authors, based on the number of patients contributed to the trial (Addendum 4).
- •
Hospital San Pedro de Alcántara, Cáceres (coordinating center)
- •
Hospital San Pablo, Barcelona
- •
Hospital General Yagüe, Burgos
- •
Hospital Arnau de Vilanova, Lérida
- •
Hospital Xeral-Calde, Lugo
- •
Hospital 12 de Octubre, Madrid
- •
Hospital La Paz, Madrid
- •
Fundación Jiménez-Díaz, Madrid
- •
Hospital Gregorio Marañón, Madrid
- •
Hospital Valdecilla, Santander
- •
Hospital San Juan, Alicante
- •
Hospital Virgen del Rocío, Seville
- •
Hospital Txagorritxu, Vitoria
- •
Hospital Miguel Servet, Zaragoza
- •
Hospital Virgen de la Macarena, Seville
At least 6 publications will be released in connection with the study. Authorship will be assigned on the basis of the percentage of patients contributed by each center, as follows:
- -
Centers contributing 10% of the study sample will designate 3 authors: 2 lead authors and 1 co-authors. The order will be established in accordance with the percentage of included subjects.
- -
Centers contributing at least 5% of the study sample will designate 3 authors: 1 lead author and 2 co-authors. The order will be established in accordance with the percentage of included subjects.
- -
Centers contributing less than 5% of the study sample will designate 1 co-author. The order will be established in accordance with the percentage of included subjects.
Before randomization, patients underwent a CPAP and NIV tolerance test. The patient was placed in a sitting position and the ventilator was set to CPAP mode at a pressure of 7cmH2O for 15min. The ventilator was then set to BIPAP mode with spontaneous breathing, using the same CPAP and an expiratory positive airway pressure (EPAP) of 16cmH2O for a further 15min. Any patients unable to adapt to ventilation were excluded, at the discretion of the investigator. Following randomization, the investigator remained with the patient for as long as it took to ensure successful adaptation, and to give the patient: (1) detailed information on the characteristics of their disease and how it can be treated with NIV, CPAP or lifestyle changes, and the importance of compliance; (2) a detailed explanation of the changes to be made in their lifestyle, on CPAP or NIV ventilators, and how to adjust and attach the mask; and (3) the short- and long-term benefits of the treatment and how it would affect their daily life.
We used the recommendations of the American Association of Sleep Medicine (AASM) for the configuration and the filter and signal settings used in study subjects. Neurological variables were electroencephalogram, electrooculogram and electromyogram readings (from the chin and both legs). Airflow measurements were obtained with nasal prongs and thermistors in PSGs with no mechanical ventilation, and from CPAP or ventilator flows in PSGs with CPAP or NIV treatment. Thoracoabdominal belt movement was measured with piezoelectric sensors or inductance. Saturation was measured by pulse oximetry (mean time between centers was 2–4s). Electrocardiogram readings were also taken, and body position was recorded. Polysomnography findings were analyzed manually by each participating center following AASM 2007 recommendations, and the sleep analysis, following the recommendations of the Spanish Sleep Group (GES).
Apnea was defined as absence of air flow (≥90% reduction) for ≥10s, and hypopnea as reduction of airflow (≥30% and ≤90%) for at least 10s, combined with ≥3% decrease in oxygen saturation or arousal.
With the patient awake, the ventilator was set to an expiratory positive airway pressure (EPAP) of between 4 and 8cmH2O and an inspiratory positive airway pressure (IPAP) of between 18 and 22cmH2O (including EPAP). Pressures were adjusted to achieve normal oxygen saturation levels (if possible), measured by pulse oximetry and patient tolerance. Breathing rate was adjusted to 12–15 breaths per minute, and tidal volume was set to 5–6ml/kg, based on actual body weight, allowing the ventilator to increase peak IPAP pressure if needed. The ventilator cycle (start, pressure and end) was re-checked to prevent asynchrony and to fine-tune the settings. After 30min of continuous ventilation, with good patient tolerance and optimal patient-ventilator interaction, a baseline arterial blood gas measurement was taken. Ventilator parameters were adjusted on the basis of PaCO2. The final adjustment was made using standard PSG: EPAP was increased to compensate for apnea, and EPAP was increased in the event of hypopnea, airflow limitation, snoring or non-apneic hypoventilation until oxygen saturation was normalized or the optimal tolerated pressure setting was reached. No changes were made to volume-assured pressure support settings during this sleep study.
Supplementary oxygen was administered if daytime PaCO2 fell to <55mmHg18; the flow rate was adjusted to maintain daytime SaO2 between 88% and 92% or PaCO2 ≥55mmHg for at least 17h/day. In patients requiring oxygen, a further blood gas measurement was taken after 20min of oxygen therapy. Supplementary oxygen was suspended if PaCO2 increased to ≥5mmHg or if pH reached <7.35.
The large sample size required called for patient recruitment at several different centers, making it difficult to conduct and coordinate the study. Nevertheless, most participating centers had already worked together on other multicenter studies, and the coordinating center has experience in coordinating similar trials.
A further difficulty was the need for a 3-year follow-up, with 2 pre-inclusion tests. The research team, however, has prior experience with 3-year follow-up studies.
Another limitation has been the absence of information in OHS literature on the incidence of cardiovascular events per year and how these might be affected by treatment, and the lack of data on the possible impact of a decrease in arterial pressure. As a result, these variables could not be factored in to sample size calculations. We believe the sample size to be adequate, but an eventual absence of statistically significant inter-group differences will not rule out the possibility of such differences arising with a larger sample size.
Mortality and the percentage of dropouts for medical reasons have been treated as operating variables, however, they will also be included in the final evaluation of study data. The sample is large enough to detect inter-group differences of 12% or more in the total sample, and of 20% or more in 30% of the sample. We believe that the sample is large enough to detect clinically relevant differences.
The design of the study was a further complication. Since severe SAHS patients cannot forego treatment (CPAP or NIV) for any prolonged period of time, randomization could only be performed once the sample had been divided into OHS patients with severe and non-severe SAHS. Because of this, 2 separate randomization processes were required to allow us to independently study 2 parallel cohorts.
Another limitation affecting sample size calculation was the absence of references in the literature to the prevalence of severe SAHS (AHI>30) in patients with OHS. Therefore, on the basis of other data and on their own experiences, the research team estimated prevalence of OHS patients without SAHS to be around 40%. The real figure, however, is nearer to 26%, and as a result, far fewer patients were recruited into the non-SAHS cohort compared to the SAHS cohort. To overcome this, the researchers decided to close recruitment to the SAHS cohort and continue to include patients in the non-SAHS cohort until 3-year follow-up finalized.
Members of the Cooperative Group are presented in Addendum 1.
Please cite this article as: López-Jiménez MJ, Masa JF, Corral J, Terán J, Ordaz E, Troncoso MF, et al. Eficacia a medio y largo plazo de la ventilación no invasiva en el síndrome de hipoventilación-obesidad (estudio Pickwick). Arch Bronconeumol. 2016;52:158–165.