Advanced kidney disease is usually considered an absolute contraindication for lung transplantation due to the difficult management of these patients in the post-operative period. Combined lung–kidney transplantation, however, could offer an opportunity for selected patients with renal and pulmonary dysfunction. This study summarizes the long-term success of a double transplantation in a 38-year-old male patient with cystic fibrosis who presented respiratory and kidney failure. After a complicated post-operative period, the patient currently lives completely independently 46months after the operation and he enjoys excellent pulmonary and renal function.
La enfermedad renal avanzada suele considerarse una contraindicación absoluta para el trasplante de pulmón, debido a la dificultad de manejo del paciente en el periodo postoperatorio, pero un trasplante combinado de pulmón-riñón podría ofrecer una oportunidad a algunos pacientes seleccionados con disfunción pulmonar y renal. En este trabajo se resume el éxito a largo plazo de un doble trasplante en un paciente varón de 38años con fibrosis quística que presentaba también insuficiencia respiratoria. Tras un periodo postoperatorio complicado, el paciente vive en la actualidad de manera completamente independiente 46meses después de la operación y disfruta de una excelente función pulmonar y renal.
The International Society for Heart and Lung Transplantation considers that advanced dysfunction of the major organs constitutes an absolute contraindication for lung transplantation.1 Nevertheless, this possibility must be considered in some cases, as patients with renal failure occasionally present other serious dysfunctions and could benefit from combined solid organ transplant. Thus, double transplantation may be indicated in some selected patients, as in the present case. Combined heart–lung and liver–lung transplants have been carried out for many years now, with good clinical results.2–4 However, very few case reports of combined lung–kidney transplantation (CLKT) have been described in the literature and, as far as we are aware, none have described this procedure in patients with end-stage renal disease on dialysis. We present the first case of CLKT successfully carried out on a dialysis patient in Spain.
Case ReportThe recipient was a 38-year-old male with cystic fibrosis (CF) who presented severe respiratory failure and end-stage renal disease. The renal dysfunction was related to long-term aminoglycoside exposure. He was dialysis-dependent and had been on hemodialysis for the previous three years. Lung transplantation was proposed in this patient (who was oxygen-dependent) in September 2008 due to pulmonary hypertension, frequent infections and multiple hospital admissions.1
Preoperative blood tests showed the following results: hematocrit 29.1%, creatinine 10.1mg/dl, urea 160mg/dl, Na 140mEquiv./l and K 5.8mEquiv./l; arterial blood gas results were as follows: pH=7.46, pO2=56mmHg and pCO2=42mmHg. Spirometry revealed an obstructive disease, with forced vital capacity (FVC) of 2690cc (50%) and forced expiratory volume in one second (FEV1) of 1480cc (30%). Sputum culture detected the presence of methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, Stenotrophomona maltophilia, Aspergillus fumigatus, Candida albicans and Candida tropicalis. Cytomegalovirus (CMV) serology was negative. The echocardiogram showed normal systolic function, mild pulmonary hypertension (mean pulmonary arterial pressure (PAP) of 47mmHg), mild tricuspid regurgitation and minimal aortic regurgitation. Chest tomography revealed the presence of diffuse bilateral bronchiectases and alveolar lesions mainly affecting the upper lobes.
The donor was a 41-year-old woman with a history of chronic hypertension who suffered a brain hemorrhage. Her last arterial blood gas showed the following values: PaO2=534mmHg with FiO2=100% and positive end-expiratory pressure (PEEP) of 5cmH2O. Endotracheal aspirate cultures and CMV serology were negative. The donor had remained in the intensive care unit (ICU) for 24h, and the explantation surgery proceeded without complications. The lungs were preserved in Perfadex* (Vitrolife, Sweden).
The recipient underwent hemodialysis immediately before surgery, and was then admitted to ICU with intubation and FiO2=100% and PEEP=10cmH2O. His hematocrit was 30%, creatinine 3.6mg/dl, Na 146mEquiv./l and K 5.7mEquiv./l. His blood gas values were as follows: pH=7.35, pO2=295mmHg and pCO2=42mmHg. Noradrenaline (0.48μg/kg/min) and nitric oxide (60ppm) were used for establishing hemodynamics in the following 48h. Immunosuppression was induced with basiliximab and maintained with tacrolimus (blood levels 10ng/ml postoperatively and 7ng/ml at 6 months), mycophenolate mofetil (1500mg/12h post-surgery, tapered to 4μg/ml in the maintenance phase) and prednisone. Corticoid levels were 30mg/day postoperatively and were gradually reduced to 15mg/day at 6 months and 10mg/day after one year.
Transverse thoracosternotomy was performed for sequential double lung transplantation, followed by kidney transplant in the right iliac fossa using the standard technique. The ischemia time was 2, 4 and 7h for the left lung, right lung and kidney, respectively.
The patient was extubated 34h after the intervention. MRSA, Pseudomonas aeruginosa, Pseudomonas mucoide, Candida albicans and Candida tropicalis were detected in the tracheal aspirates, but the samples were negative for CMV. Intravenous (linezolid, voriconazole, trimetoprim-sulfametoxazole and piperacillin/tazobactam) and inhaled antibiotic treatment (tobramycin and amphotericin lipid complex) was instigated.
Progressive deterioration in respiratory function, with CO2 retention, resulted in reintubation on day 10 post-surgery. Acute lung rejection was suspected and corticosteroids were administered for three days. The patient presented anuria during the immediate postoperative period and required continuous venovenous hemodialysis for 25 days. Renal Doppler ultrasound showed permeable blood vessels. The first renal biopsy (with a serum tacrolimus level of 13ng/ml) showed mild interstitial fibrosis, mild atherosclerosis, moderate acute tubular necrosis, absence of glomerular pathology and weakly positive for CD4 in the peritubular capillaries. Renal function gradually recovered, and the creatinine clearance was 42ml/min 2 months after surgery. On day 50 post-surgery, the patient presented abdominal pain associated with leukocytosis. Acute cholecystitis was diagnosed and the patient underwent a cholecystectomy with peritoneal lavage, after which an urgent laparotomy revealed the presence of a perforated gangrenous gall bladder, with associated choleperitonitis.
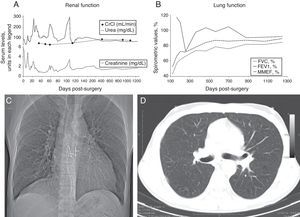
The patient was discharged 80 days post-transplant, with improvement in the spirometry and renal function (Fig. 1A and B).
Forty-two months after the DLKT, the patient was asymptomatic and leading an active life. The chest X-ray did not show any abnormal findings (Fig. 1C) and the FEV1 was 3340cc (84.5%); serum creatinine was 1.3mg/dl, and the creatinine clearance 86ml/min.
DiscussionAccording to clinical guidelines for lung transplantation, CF is the third most common indication for which this procedure is performed, but the multisystem nature of the disorder poses additional difficulties in the selection of candidates.1 These patients often present chronic infections due to antibiotic-resistant microorganisms which remain in the airways and sinuses after the transplant; in the context of immunosuppression, this constitutes a source of possible lung infections.5 Although nephropathy is rare in CF, this disorder shows some abnormalities in renal function, and various drugs are used in the treatment of CF and infections that may be nephrotoxic, such as the aminoglycosides.6,7
The first case of a double lung–kidney transplant was published in 1998 in a patient with pulmonary lymphangioleiomyomatosis and renal angiolipomas after a unilateral nephrectomy. This patient had acceptable creatinine clearance, so a possible postoperative deterioration in renal function could be avoided.2 No similar cases were subsequently published, and although the International Society of Heart and Lung Transplantation database includes cases of patients treated with combined transplantation of a single lung–kidney, there are no data on their preoperative renal function or postoperative survival.
Simultaneous solid organ transplant has been more common in recent years, and attempts have been made in reviews of combined liver–kidney transplantation3 and combined heart–lung transplantation8 to analyze the indications, technical considerations and expected results. Rana et al.9 recently published the description of a simultaneous combined heart–lung–kidney transplantation with satisfactory results, and highlighted the results of a previous study, in which the simultaneous transplantation of multiple organs from the same donor showed lower rejection rates than single organ transplantation.
CLKT is a surgically viable intervention at present, but postoperative patient management may be difficult, given that the strict fluid restriction required to prevent pulmonary edema must be balanced with the need for abundant fluid intake for renal function. Furthermore, immunosuppressive treatment for lung transplantation must be optimized to reduce the nephrotoxic effects, especially when anti-calcineurin drugs are used.10 In our case, the required dose of calcineurin inhibitors was reduced by using basiliximab for the induction phase and tacrolimus/mycophenolate instead of cyclosporine/azathioprine for the maintenance phase.
In conclusion, CLKT may be indicated in patients who are candidates for lung transplantation with concomitant end-stage renal disease. This procedure is surgically viable, but perioperative patient management is complex. To obtain good results, CLKT should only be carried out in specially trained centers with adequate donor and recipient selection.
Conflict of InterestThe authors do not have any conflicts of interest.
Please cite this article as: Borro JM, et al. Éxito a largo plazo de un trasplante combinado de pulmón-riñón en un paciente con fibrosis quística. Arch Bronconeumol. 2013;49:272-4.