Endobronchial ultrasound (EBUS) is a new technique that can be used for the diagnostic imaging of central pulmonary thromboembolism (PE). In eight cases at our clinic, EBUS was used because of mediastinal lymphadenopathies or paramediastinal nodular lesions and at the same time images of a PE were obtained by means of EBUS. The PE was diagnosed before the EBUS with computed tomography (CT) of the lungs in all cases (5 women and 3 men). The repletion defects of all the cases compatible with a PE were clarified with CT-angiography. EBUS may be an alternative method for the diagnosis of PE, since it can indicate the presence of a thrombus in the central pulmonary arteries in hemodynamically stable cases.
La ecografía endobronquial (EBUS) es una nueva técnica que puede utilizarse para el diagnóstico por la imagen del tromboembolismo pulmonar (TEP) central. En 8 casos de nuestra clínica se utilizó la EBUS a causa de adenopatías mediastínicas o lesiones nodulares paramediastínicas, y al mismo tiempo se obtuvieron imágenes de un TEP mediante la EBUS. El TEP se diagnosticó antes de la EBUS mediante tomografía computarizada (TC) pulmonar en todos los casos (5 mujeres y 3 varones). Los defectos de repleción de todos los casos compatibles con un TEP se esclarecieron mediante angio-TC. La EBUS puede ser un método alternativo para el diagnóstico del TEP, ya que puede indicar la presencia de un trombo en arterias pulmonares centrales en casos en los que la hemodinámica es estable.
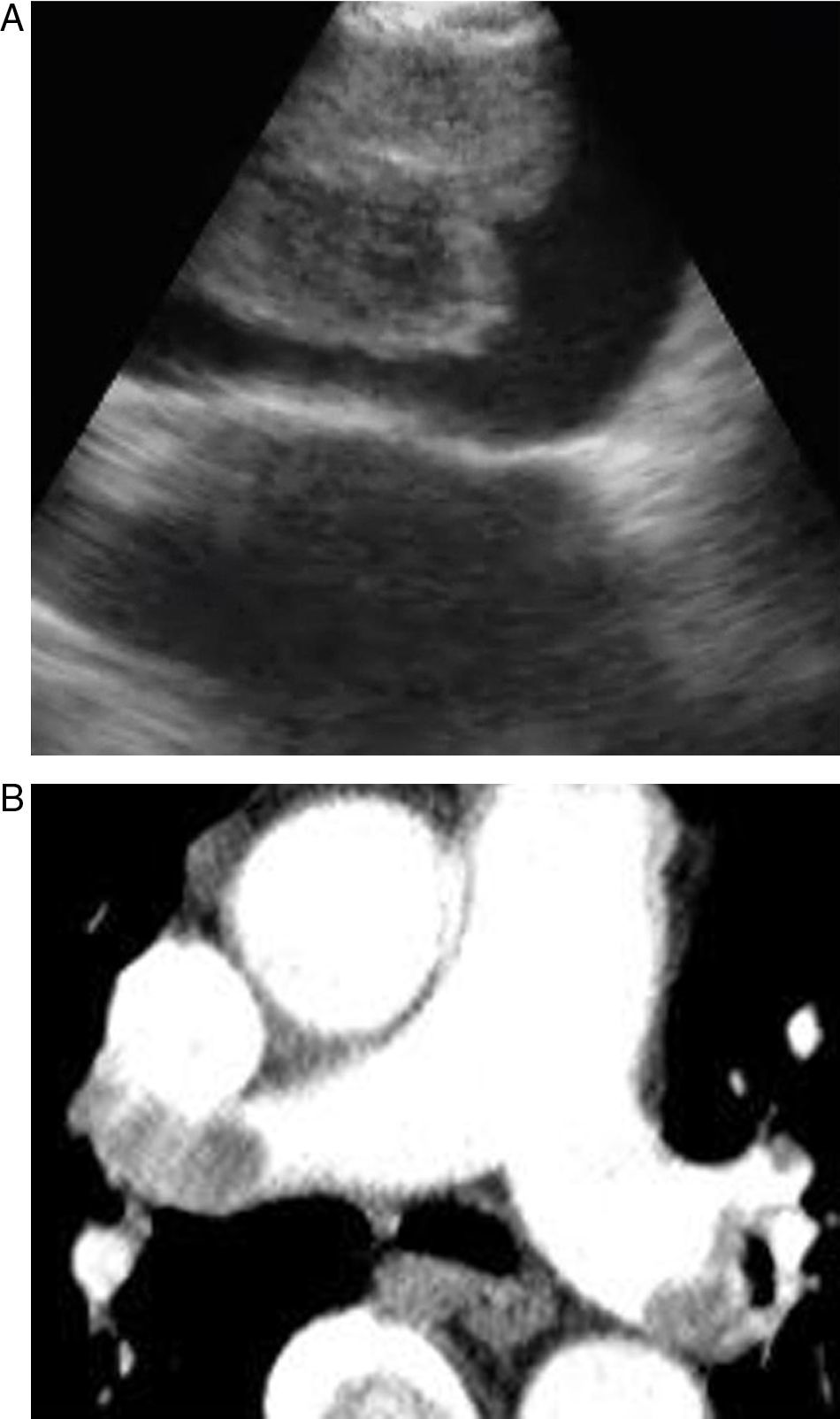
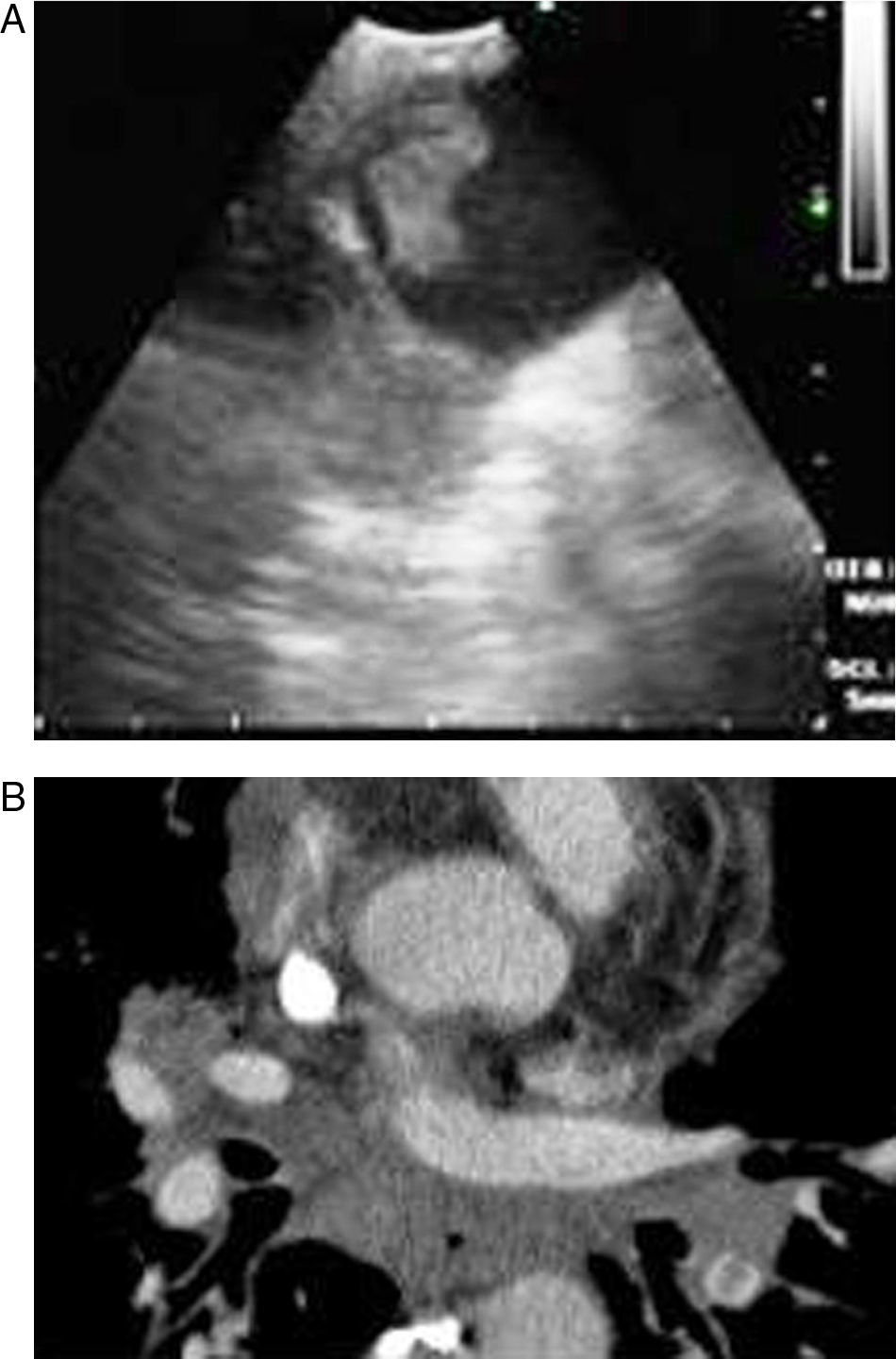
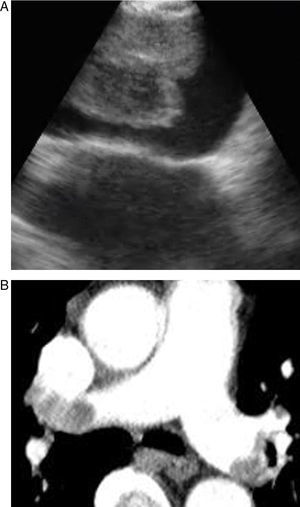
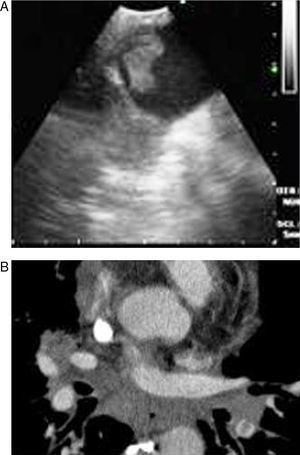
Pulmonary thromboembolism (PE) is a medical disorder related with sudden occlusion of the pulmonary artery. It has a high mortality and morbidity rate. PE can be erroneously diagnosed in patients with comorbidities. The systematic use of multislice computed tomography (CT) angiography of the chest is the technique of choice for the diagnosis of PE. The use of contrast solutions and radiation are its main limitations in cases of renal failure and in pregnancy.1,2 Therefore, other diagnostic tests are essential in these situations (Figs. 1 and 2).
Endobronchial ultrasound (EBUS) is a new diagnostic technique in pulmonary medicine. By using ultrasound on the tip of the bronchoscope, the tissue surrounding the respiratory system can be evaluated and invasive techniques applied. The main indications for EBUS are mediastinal lymphadenopathies and central masses. Its use enables both histopathological diagnosis of the mass (malignant disease, tuberculosis, sarcoidosis) and staging of malignant disease to be easily determined3 (Tables 1 and 2).
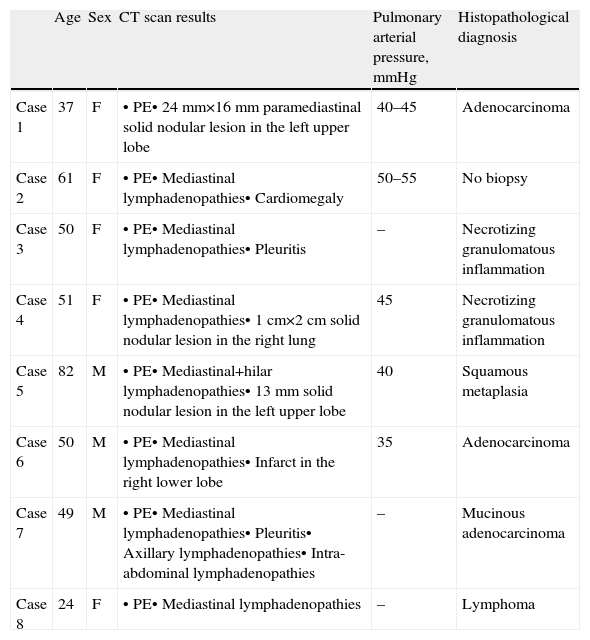
Patient Characteristics.
| Age | Sex | CT scan results | Pulmonary arterial pressure, mmHg | Histopathological diagnosis | |
| Case 1 | 37 | F | • PE• 24mm×16mm paramediastinal solid nodular lesion in the left upper lobe | 40–45 | Adenocarcinoma |
| Case 2 | 61 | F | • PE• Mediastinal lymphadenopathies• Cardiomegaly | 50–55 | No biopsy |
| Case 3 | 50 | F | • PE• Mediastinal lymphadenopathies• Pleuritis | – | Necrotizing granulomatous inflammation |
| Case 4 | 51 | F | • PE• Mediastinal lymphadenopathies• 1cm×2cm solid nodular lesion in the right lung | 45 | Necrotizing granulomatous inflammation |
| Case 5 | 82 | M | • PE• Mediastinal+hilar lymphadenopathies• 13mm solid nodular lesion in the left upper lobe | 40 | Squamous metaplasia |
| Case 6 | 50 | M | • PE• Mediastinal lymphadenopathies• Infarct in the right lower lobe | 35 | Adenocarcinoma |
| Case 7 | 49 | M | • PE• Mediastinal lymphadenopathies• Pleuritis• Axillary lymphadenopathies• Intra-abdominal lymphadenopathies | – | Mucinous adenocarcinoma |
| Case 8 | 24 | F | • PE• Mediastinal lymphadenopathies | – | Lymphoma |
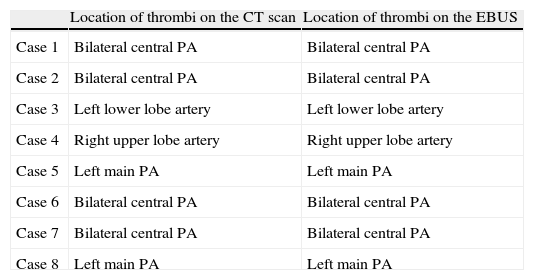
Identification of Thrombi by CT Scan and by EBUS.
| Location of thrombi on the CT scan | Location of thrombi on the EBUS | |
| Case 1 | Bilateral central PA | Bilateral central PA |
| Case 2 | Bilateral central PA | Bilateral central PA |
| Case 3 | Left lower lobe artery | Left lower lobe artery |
| Case 4 | Right upper lobe artery | Right upper lobe artery |
| Case 5 | Left main PA | Left main PA |
| Case 6 | Bilateral central PA | Bilateral central PA |
| Case 7 | Bilateral central PA | Bilateral central PA |
| Case 8 | Left main PA | Left main PA |
PA: pulmonary artery.
Some articles have recently been published on the use of EBUS as a diagnostic method for central PE.4–6 In this paper, we evaluated PE in eight patients using EBUS, and have assessed the importance of this technique in the diagnosis of PE.
Clinical FindingsCase 1A 37-year-old woman attended the emergency department with cough and hemoptysis. The chest computed tomography (CT) scan showed a 24mm×16mm solid nodular paramediastinal lesion in the left upper lobe, and the bilateral presence of a thrombus in the pulmonary arteries. Endobronchial ultrasound-guided fine needle aspiration (EBUS-FNA) was used to diagnose the solid nodular lesion, and a PE was observed in the bilateral central pulmonary arteries.
Case 2A 61-year-old woman underwent surgery for a meniscal tear. After the operation, she suffered a massive pulmonary embolism and thrombolytic treatment was applied. Mediastinal lymph nodes were also observed on the chest CT scan. Six days after thrombolytic treatment, the patient was hemodynamically stable, and EBUS was used to examine the lymph nodes. Since the images of the lymph nodes had benign echographic characteristics, FNA was not performed. During the EBUS, the presence of bilateral pulmonary arterial thrombi was observed.
Case 3A 50-year-old woman attended the emergency department for sudden onset of dyspnea and fatigue. The chest CT scan revealed the presence of a thrombus in the left lower lobe artery, as well as 2cm mediastinal, hilar and subcarinal lymph nodes. EBUS-FNA of the nodes was performed; during the EBUS, the thrombus was seen in the left lower lobe artery. Caseous necrosis was diagnosed in the histopathological examination.
Case 4A 51-year-old woman attended hospital for dyspnea and chest pain. The chest CT scan revealed the presence of a 2cm solid nodular lesion in the right lung, mediastinal lymphadenopathies and a PE in the right upper lobe artery. EBUS-FNA of the lesion and lymph nodes was performed; during the EBUS, the thrombus was seen in the right upper lobe artery. Necrotizing granulomatous inflammation was identified in the histopathological examination.
Case 5An 82-year-old man attended hospital for dyspnea and chest pain. The chest CT scan revealed a 13mm solid nodular lesion in the left upper lobe, together with mediastinal and hilar lymphadenopathies and a PE in the left main pulmonary artery. EBUS-FNA of the lesion and lymph nodes was performed; during the EBUS, the thrombus was seen in the left main pulmonary artery. Squamous metaplasia was observed in the histopathological examination.
Case 6A 50-year-old male attended the emergency department for hemoptysis and chest pain. The chest CT scan revealed an infarct in the right lower lobe, together with mediastinal lymphadenopathies and a bilateral PE in the central pulmonary arteries. EBUS-FNA of the lymph nodes was performed; during the EBUS, a thrombus was seen in the bilateral central pulmonary arteries. The histopathological examination indicated the presence of an adenocarcinoma.
Case 7A 49-year-old male attended the emergency department for hemoptysis, chest pain and neck swelling. The chest CT scan revealed mediastinal, axillary lymphadenopathies, minimal bilateral pleuritis and a PE in the bilateral central pulmonary arteries. EBUS-FNA of the lymph nodes was performed; during the EBUS, a thrombus was seen in the bilateral central pulmonary arteries. Mucinous adenocarcinoma was observed in the histopathological examination.
Case 8A 24-year-old woman attended hospital with a one-month history of cough and chest pain. Multiple lymph nodes and a thrombus in the superior vena cava were observed on the chest CT scan. EBUS-FNA was used for the diagnosis. During the EBUS examination, in addition to the thrombus in the superior vena cava, the presence of a thrombus in the right upper pulmonary artery was also observed. Histopathological examination of the lymph node biopsies indicated the presence of a B-cell lymphoma.
DiscussionPE is a common lung condition which is difficult to diagnose, and is the leading life-threatening pulmonary emergency. Patients with PE usually experience sudden pleuritic chest pain with breathing difficulties, but sometimes may have non-specific symptoms that can cause the diagnosis of PE to be delayed or indeed not established at all. This delay in diagnosis is the most significant factor in PE mortality. Thus, patients in whom PE is suspected must be evaluated and diagnosed quickly. There are no accurate non-invasive tests.1,2 Multislice CT angiography of the chest has recently emerged as the method of choice for the diagnosis of PE. The increased number of detectors enables peripheral thrombi to be easily visualised.7 However, the radiation dose increases with the number of detectors, examination area and thickness of the slice. Furthermore, an iodated contrast solution, which is nephrotoxic, is required for the angiography. As a result, CT angiography is not an appropriate method in pregnant women, patients with renal failure and patients allergic to contrast, so other diagnostic tests must be used to establish the presence of a PE. According to the PIOPED II study, CT angiography cannot be used in 24% of patients with PE due to contraindications.2 Ventilation/perfusion (V/Q) scintigraphy is another diagnostic test used for PE. Radioactive solutions must also be used for this examination. V/Q scintigraphy is appropriate in patients in whom PE is suspected, and who have a normal chest X-ray and normal basic cardiopulmonary characteristics. If the chest X-ray is abnormal, the diagnostic value of the scintigraphy is reduced.8 Magnetic resonance imaging can also be used to visualize the thrombus in the pulmonary artery; movement artifacts, allergy to gadolinium and inapplicability in emergency situations are its main disadvantages.9 Approximately 70% of patients with PE have deep vein thrombosis (DVT). The diagnosis of DVT is important information for initiating anticoagulant treatment in patients in whom PE is suspected. However, discarding DVT does not discard the presence of a PE. There are no definitive diagnostic tests with minimal complications that can be applied in all cases. Consequently, many diagnostic tests must be used in combination.1,2
EBUS is generally used for the biopsy of mediastinal and hilar lymphadenopathies or masses. The pulmonary arteries are around 5mm in the tracheobronchial zone. Thus, EBUS can visualize them easily. We used a convex ultrasound probe (CP) (Olympus EBUS-CV-180, Tokyo, Japan) for the bronchoscopic evaluation. The external diameter of the CP-EBUS is 6.9mm. It has a 2.0mm working channel and a 30 degree oblique anterograde viewing optic. The maximum penetration of the linear ultrasound transducer is 50mm, and it is connected to a processor that enables the flow of the mediastinal and hilar vessels to be visualised.10
Casoni et al. used the EBUS in a patient who presented a suspicious low density intra-arterial lesion on the pulmonary angiography, and for the first time published the diagnosis of a PE.4 Aumiller et al. incidentally diagnosed PEs in three patients during EBUS examinations to stage lung cancer. A multicenter study was later started to evaluate the presence of PE using CP-EBUS in the first 24h following a diagnosis of PE by CT angiography. Thrombi were easily detected in the pulmonary arteries in 28 out of 32 patients. A total of 97 of the 101 thrombi diagnosed using CT angiography were confirmed with EBUS (96% sensitivity). EBUS enables the pulmonary trunk, main pulmonary arteries and lobar arteries to be visualized. One of the four thrombi that could not be visualized was in the middle lobe artery, and three were in the left upper lobe artery. None of the patients suffered any complications during the study. As a result, the authors reached the conclusion that EBUS was a feasible diagnostic tool for detecting central PE.5 Following this study, many cases were published describing the use of EBUS in the diagnosis of PE.6,11–13
In our study, all patients were hemodynamically stable and were sedated during the bronchoscopy. EBUS was carried out using an oral approach, with conscious sedation in all patients. Arterial blood pressure, pulse rate and oxygen saturation were constantly recorded during all the procedures. The main pulmonary arteries were visualized after evaluating the mediastinal lymphadenopathies in all cases. The bronchoscopist knew the location of the filling defects observed in the pulmonary arteries on the CT scan. Permeable pulmonary arteries did not show echoes. In our cases, when echogenic zones were visualized, the bronchoscopist confirmed the presence of the thrombus using the Doppler mode.
In conclusion, we evaluated PE using EBUS easily and reliably. PE guidelines do not refer to EBUS in relation to the diagnosis of PE. Since CT angiography cannot be applied in a significant number of patients, EBUS-guided imaging diagnosis of the thrombi could be a good alternative in hemodynamically stable patients for the diagnosis of PE. Furthermore, in intensive care units, transferring some patients to the CT unit is a major problem, so EBUS could be useful in these cases for diagnosing PE. Moreover, EBUS can visualize the central vasculature and enable vascular malignant diseases to be easily differentiated from PE. However, additional single-blind, randomized, controlled studies will be necessary, since in our study, as well as in the one by Aumiller et al., the bronchoscopists knew the location of the PE in the CT angiography.5
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Şentürk A, et al. Diagnóstico por la imagen del tromboembolismo pulmonar mediante ecografía endobronquial. Arch Bronconeumol. 2013;49:268-71.