Autonomic dysfunction can alter heart rate variability and increase the incidence of arrhythmia. We analyzed the impact of continuous positive airway pressure (CPAP) on this pathophysiological phenomenon in patients with severe sleep apnea–hypopnea syndrome.
MethodsConsecutive patients with recently diagnosed severe sleep apnea–hypopnea syndrome were prospectively considered for inclusion. Incidence of arrhythmia and heart rate variability (recorded on a 24-h Holter monitoring device) were analyzed before starting CPAP therapy and 1 year thereafter.
ResultsA total of 26 patients were included in the study. CPAP was administered for 6.6±1.8h during Holter monitoring. After starting CPAP, we observed a marginally significant reduction in mean HR (80±9 to 77±11bpm, P=.05). CPAP was associated with partial modulation (only during waking hours) of r-MSSD (P=.047) and HF (P=.025) parasympathetic parameters and LF (P=.049) sympathetic modulation parameters. None of these parameters returned completely to normal levels (P<.001). The number of unsustained episodes of atrial tachycardia diminished (P=.024), but no clear effect on other arrhythmias was observed.
ConclusionsCPAP therapy only partially improves heart rate variability, and exclusively during waking hours, and reduces incidence of atrial tachycardia, both of which can influence cardiovascular morbidity and mortality in sleep apnea–hypopnea syndrome patients.
La disfunción del sistema nervioso autonómico produce alteraciones en la variabilidad de la frecuencia cardiaca y aumenta la incidencia de arritmias. Analizamos este fenómeno fisiopatológico en pacientes con síndrome de apnea/hipoapnea del sueño severo y el impacto sobre el mismo del tratamiento con presión positiva continua en la vía aérea (CPAP).
MétodosPacientes consecutivos con síndrome de apnea/hipoapnea del sueño severo de reciente diagnóstico fueron prospectivamente considerados para inclusión. Se analizó la incidencia de arritmias y la variabilidad de la frecuencia cardiaca (obtenidos mediante registro Holter de 24horas) antes de iniciarse tratamiento con CPAP y tras un año del mismo.
ResultadosSe incluyeron 26 pacientes. El tiempo de uso de CPAP durante el registro Holter fue de 6,6±1,8 horas. Tras inicio de CPAP, se apreció una reducción marginalmente significativa en la FC media (80±9 a 77±11 lpm, p=0,05). El uso de CPAP se asoció a una modulación parcial y exclusivamente en horas de vigilia de los parámetros de modulación parasimpática r-MSSD (p=0,047) y HF (p=0,025) y de modulación simpática LF (p=0,049). Ninguno de estos revirtió completamente a la normalidad (p<0,001). Se observó una reducción de los episodios no sostenidos de taquicardia auricular (p=0,024), sin efecto demostrativo sobre otras arritmias.
ConclusionesEl tratamiento con CPAP se asocia a una mejora solo parcial y diurna de la variabilidad de la frecuencia cardiaca y disminuye la incidencia de taquicardia auricular. Ambos efectos podrían influir en la morbimortalidad cardiovascular de los pacientes con síndrome de apnea/hipoapnea del sueño.
Changes in the autonomic nervous system are associated with diminished tachycardia and bradycardia responses to sympathetic and parasympathetic stimuli, reducing heart rate variability (HRV). This pathophysiological phenomenon is also associated with increased atrial and ventricular arrhythmias. HRV changes and the incidence of arrhythmias are associated with increased cardiovascular morbidity and mortality, particularly in patients with underlying heart disease.1–4
Imbalances between the parasympathetic or vagal nervous system and the sympathetic nervous system in sleep apnea–hypopnea syndrome (SAHS) are due to 2 mechanisms. One involves a disruption of the physiological increase in vagal tone during the transition from waking to sleep. In the other, hypoxia and recurrent arousals induce sympathetic hyperactivation and parasympathetic inhibition.5 As a result of this pathophysiological process, heart rate (HR) modulation – particularly its vagal component – is compromised during sleep in patients with SAHS, and this alteration can continue during waking hours.6
Continuous positive airway pressure (CPAP) in the upper airways improves blood pressure control, and has a positive impact on the cardiovascular prognosis of patients with structural heart disease.5–7 CPAP may correct the influence of the vagal response on HRV and reduce sympathetic hyperactivity.8–10 However, there is scant evidence that CPAP has a continuous, positive effect on HRV and the incidence of arrhythmias, and what little is available is sometimes controversial.8–20
In this study, we analyzed the hypothesis that autonomic dysfunction attributed to SAHS can be characterized by 24-h Holter monitoring (primary objective) and that CPAP treatment has a modulatory effect on this phenomenon (secondary objective). We analyzed the behavior of 2 cardiovascular phenomena that are a direct consequence of an altered autonomic tone: HRV and the incidence of arrhythmias.
MethodsStudy PopulationPatients were recruited between January and March 2013 in the outpatient clinic of the Respiratory Sleep Disorders Unit of our hospital. In the initial visit, information relating to sleep disorders was collected (chronic snoring, apneas, excessive daytime sleepiness evaluated using the Epworth test, and cardiovascular history). Anthropometric parameters, such as body mass index (BMI) and neck circumference were also recorded. Patients then underwent polysomnography and lung function test. Patients who met any of the following criteria were excluded: daytime respiratory failure, severe heart failure, CPAP, home oxygen therapy, non-invasive mechanical ventilation, known history of arrhythmias, and underlying chronic obstructive lung disease (for which an independent association with altered HRV has been reported, in order to avoid bias in measures derived from the presence of overlap syndrome). Consecutive patients with a diagnosis of severe SAHS who did not present any of the exclusion criteria were included prospectively in the study, after signing informed consent. The study was approved by the Ethics Committee of our hospital.
Polysomnography and CPAPThe sleep study was performed using a monitored respiratory polygraph (eXea series 5; Bitmed, Zaragoza, Spain). The recordings were read manually, based on the definitions of respiratory events proposed by the American Academy of Sleep Medicine. The average number of apneas and hypopneas per hour was taken as the apnea–hypopnea index. The percentage of time with oxygen saturation <90% was identified as CT90%. According to the latest SEPAR guidelines, CPAP was indicated in patients with an apnea–hypopnea index ≥30/h, even in those with mild symptoms.21
CPAP therapy was initiated at an empirical pressure of 8cmH2O for 1 month. Subsequently, when tolerance to treatment was considered appropriate, optimal pressure was applied with auto-CPAP titration (S9 AutoSet, ResMed).22
Cardiology EvaluationThe cardiology evaluation included a 12-lead electrocardiogram (ECG), a 2D echocardiogram, and a 24-h Holter recording (Cardioscan II®, DM Software, Nevada, US) before starting CPAP treatment, and after 1 year of treatment. These recordings were examined for incidence of atrial and ventricular arrhythmias that could potentially affect cardiovascular morbidity and mortality, including frequent ventricular extrasystoles (VE) (>1% of total beats), periods of sustained or unsustained atrial (AT)/ventricular tachycardia (VT) (>30s) and episodes of atrial fibrillation (AF). Arrhythmias with no deleterious impact on cardiovascular prognosis were excluded from the analysis (e.g., atrial extrasystoles and atrioventricular re-entrant paroxysmal supraventricular tachycardia). The total number of AT/AF/VT episodes and beats before CPAP and 1 year after CPAP initiation was calculated. Patients in persistent AF or with excessively frequent VEs (>20% of total beats) were excluded to avoid bias in the analysis of HRV derived from the absence of normal sinus rhythm.
Mean HR and HRV over a 24-h period (and separately for periods of waking and sleep) were determined on Holter recordings. Mean HR is liable to high intra- and inter-individual variability, so HRV is a better indicator of how HR responds to the vagal autonomous system (that reduces HR) and/or the sympathetic system (that increases HR). Altered HRV parameters, therefore, indicate an altered autonomic response. The following HRV variables were determined: standard deviation of beat-to-beat (NN) intervals (SDNN); standard deviation of average NN intervals (SDANN); mean of SDNN in 5min (SDNN index); root mean square of successive differences (r-MSSD); proportion of NN50 divided by total number of NNs (pNN50); absolute value of components with very low frequency (VLF); low frequency (LF); and high frequency (HF) of the total HR spectrum and the LF/HF index.6 More simply, the expression of the parasympathetic or vagal modulation of the HR are essentially the parameters r-MSSD, HF and LF/HF, the most indicative of sympathetic modulation of HR being the parameter LF.6
The HRV parameters obtained were compared with previously established reference values for a healthy adult population.23
Follow-UpPatients were examined and underwent a 12-lead ECG 6 and 12 months after inclusion in the study. The use of drugs that could potentially affect HRV and the rate of arrhythmias, including beta-blockers, betamimetics and anti-arrhythmic agents was discouraged during the trial. During the study, adherence to CPAP therapy was calculated with remote monitoring. Patients with a mean CPAP compliance of less than 3h and less than 4h during Holter monitoring were excluded from the study.
Statistical AnalysisChanges in incidence of arrhythmias and HRV associated with the use of CPAP were analyzed using the t-test for dependent paired variables. Distribution was not normal, so the parameters HRV, VLF, LF, and HF were transformed using logarithmic techniques.8 A t-test for independent variables was performed to compare HRV values with reference values.23 Categorical variables were compared using Fisher's test. All analyses were performed using the SPSS 18.0 statistical software package.
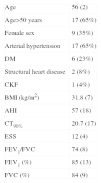
ResultsStudy Population. Baseline CharacteristicsAfter exclusion of 2 patients who did not comply adequately with CPAP, a total of 26 patients were studied. Characteristics of the study population are described in Table 1. It is interesting to note that while most patients had hypertension (17 patients), only a minority had underlying heart disease (2 patients with coronary disease). None of the patients received antiarrhythmics, beta-blockers or betamimetics during the study period, with the exception of the 2 patients with coronary disease, who were receiving betablockers. CT90% was significantly elevated in 8 patients (>30%).
Clinical Characteristics of Study Population.
| Age | 56 (2) |
| Age>50 years | 17 (65%) |
| Female sex | 9 (35%) |
| Arterial hypertension | 17 (65%) |
| DM | 6 (23%) |
| Structural heart disease | 2 (8%) |
| CKF | 1 (4%) |
| BMI (kg/m2) | 31.8 (7) |
| AHI | 57 (18) |
| CT90% | 20.7 (17) |
| ESS | 12 (4) |
| FEV1/FVC | 74 (8) |
| FEV1 (%) | 85 (13) |
| FVC (%) | 84 (9) |
Values are expressed as mean (standard deviation) or proportion (percentage).
AHI, apnea–hypopnea index; BMI, body mass index; CKF, chronic kidney failure (defined as creatinine clearance<60mL/min); CT90%, percentage of night time with oxygen saturation <90%; DM, diabetes mellitus; ESS, Epworth Sleepiness Scale score; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity.
Left ventricular ejection fraction was 62±2%, with a left atrial diameter of 40±5mm and estimated pulmonary systolic pressure of 30±4mmHg.
Impact of CPAP on Heart Rate VariabilityPatients used CPAP for 6.6±1.8h during the Holter recording and 5.3±2h during the study period.
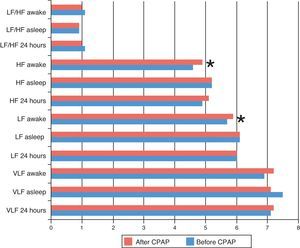
Comparable parameters were lower than reference values in our population, including SDNN (P=.003), SDANN (P=.017), LF (P<.001) and HF (P<.001), with the exception of rMSSD (P=.13). After using CPAP, none of the parameters completely normalized, compared to these reference values (P=.009, P=.02, P<.001 and P<.001, respectively), but only HF remained below the lowest expected cut-off point (1.96 times the standard deviation, P<.001).
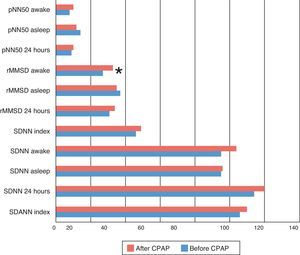
After starting CPAP, improvements were only seen in rMSSD (P=.047), LF (P=.049) and HF (P=.025), exclusively during waking hours. No significant effect was seen during night-time sleep or in other parameters (Figs. 1 and 2).
HRV time-domain parameters before and after continuous positive airway pressure. CPAP, continuous positive airway pressure; pNN50, proportion of NN50 divided by total number of beat-to-beat intervals; (rMSSD), root mean square of successive differences; SDANN, standard deviation of average beat-to-beat intervals; SDNN index, mean of SDNN in 5min; SDNN, standard deviation of beat-to-beat intervals. *P<.05 compared before and after 1 year of CPAP.
CPAP, therefore, did not modulate any HRV parameters during sleep, in spite of good compliance.
Changes in Incidence of ArrhythmiasMean HR at inclusion was 80±9bpm. One year after starting CPAP, we observed a marginally significant reduction to 77±11bpm (P=.05).
No sustained episodes of AT, VT or AF were registered. Findings consisted mainly of occasional VE, unsustained episodes of AT, and (in 2 patients only) VT, and inappropriate sinus tachycardia (Table 2).
Arrhythmias Before and After Starting CPAP.
| Arrhythmias | Before CPAP | After CPAP | P* |
|---|---|---|---|
| Sinus dysfunction | 2/26 | 1/26 | 1 |
| 2nd/3rd degree AVB | 2/26 | 1/26 | 1 |
| NSAT | 8/26 | 1/26 | .024 |
| Number of episodes of NSAT, mean (SD) | 10 (27) | 0.3 (1) | .15 |
| Maximum number of NSAT beats, mean (SD) | 4 (3) | 1 (1) | .026 |
| NSVT | 2/26 | 0/26 | .49 |
| VE, mean (SD) | 190 (422) | 276 (374) | .44 |
Values presented as proportion or mean [standard deviation (SD)].
AVB, atrioventricular block; NSAT, non-sustained atrial tachycardia; NSVT, non-sustained ventricular tachycardia; VE, ventricular extrasystoles.
CPAP treatment was associated with a reduction in the number of patients with unsustained AT (from 8 to 1, P=.024) and in the total number of AT beats (P=.026). In contrast, the number of VEs did not fall significantly (P=.44).
Two patients developed episodes of second-degree atrioventricular block. The use of CPAP in both patients resolved conduction changes and neither required pacemaker implantation. Two more patients had non-severe sinus dysfunction, which resolved in 1 of them after introduction of CPAP; neither required a pacemaker.
Subgroup AnalysisPatients over 50 years of age (n=17) had more disturbed baseline HRV figures than younger patients (P<.05) and their frequency domain parameters (VLF, LF and HF) during waking hours improved more significantly than the under-50 age group (P<.05). Age did not affect the incidence of arrhythmias, nor the influence of CPAP on this parameter. Arterial hypertension was more common in patients aged>50 years (14/17 vs. 3/9 patients, P=.03), and these hypertensive patients tended to have more altered initial HRV figures and to achieve greater improvement after receiving CPAP (P<.05). Patients’ sex, BMI and presence of diabetes mellitus (DM) did not produce differences in HRV and incidence of arrhythmias nor in the effect of CPAP on these parameters
DiscussionIn this study, we found that patients with SAHS have HRV values suggestive of autonomic dysfunction, and that CPAP therapy only partially improves HRV, and exclusively during waking hours, as indicated by an increase in LF (P=.049) and HF (P=.025) parameters. This effect particularly concerns the HF parameter, the most direct indicator of the response of HR to the vagal stimulus, which remains below the minimum cut-off point (P<.001). In our series, in which only a minority of patients had heart disease, CPAP reduced the number of episodes of non-sustained AT and the number of patients with this arrhythmia (P=.026 and P=.024, respectively). No reduction in the VE burden could be demonstrated after CPAP; nor did CPAP have any effect on the reduction of sustained AT and VT episodes or AF (underrepresented in our series).20
The positive effect of CPAP on HRV appears to be particularly pronounced in patients over 50 years of age. However, this finding must be interpreted with caution, since a possibly greater CPAP effect on autonomic modulation in patients over 50 years of age may, in our analysis, be determined by more altered baseline HRV figures in this subpopulation, and by the lower proportion of individuals younger than 50 years in our series (only 9 of 26 patients).
CPAP is Associated With Improved Daytime But Not Night-time Heart Rate VariabilityIt is interesting to note that none of the time domain parameters improved after CPAP, with the single exception of rMSSD (considered an equivalent of HF). The reason for this apparent absence of effect on the time domain parameters may be that these parameters are less specific for sympathetic or parasympathetic activity. Moreover, these measures use a possibly excessive spectrum of beats during the Holter recording, which may be unsuitable for analyzing the impact of this therapy on HRV.6
We find it surprising that CPAP fails to affect the HF and LF parameters (the most representative of parasympathetic and sympathetic modulation) during the night, precisely at the time when this therapy is being applied. This issue merits a more detailed discussion. The HF and LF parameters are only analyzed at the lower and upper ends of the HR spectrum, thus incorporating only a small proportion of the total number of beats. The number of beats is even lower during sleep, when abrupt and extreme changes in HR are less common. As a consequence of this physiological condition, HRV during sleep (and therefore the parameters HF and LF) is less than during waking hours. Patients with SAHS have an abnormally high sympathetic tone during sleep, which increases mean HR and contributes to an additional reduction of HRV, diminishing the possibility of CPAP-induced changes in HF and LF parameters at night.
The analysis of night-time HRV in SAHS patients may depend on the influence of altered breathing patterns.11,16 This may not only explain some discrepancy in results among the different studies, it may also mean that changes in HRV during sleep in these patients are misinterpreted. It seems that the association between long-term CPAP and improved HRV is most evident during waking hours, when HRV is less influenced by breathing patterns.
The characteristic increase in sympathetic tone and loss of parasympathetic tone during sleep in SAHS patients is known to persist to some degree during waking hours. This is a consequence of increased catecholamine secretion and chemo- and baroreceptor saturation.24 CPAP reduces this traffic and thus the sympathetic tone, thus enhancing the physiological response to sympathetic and vagal stimuli during waking hours.15,24 This phenomenon would explain the daytime changes seen in HRV, revealed in a novel way in this study by using 24-h Holter.
Previous StudiesResults from studies analyzing the impact of CPAP on HRV are far from homogeneous.8–15 These apparent discrepancies may be due to methodological problems, such as the use of 5-min ECG fragments during specific sleep phases (fundamentally during stage 2 non-REM sleep), instead of continuous ECG recordings. Moreover, very few studies have analyzed the long-term impact of CPAP treatment on HRV. Our findings are similar to previous data which suggest a reduction in daytime sympathetic tone, a physiological effect that contributes to increased HRV during waking hours.15 In addition to this effect on daytime sympathetic tone, we also found, in line with earlier studies,8 that the daytime response to parasympathetic or vagal stimuli normalizes after starting CPAP. The possibility that a long-term improvement in HRV confers a better cardiovascular prognosis to patients without other comorbidities (hypertension, heart failure or structural heart disease) is still unproven. Our study and others suggest that CPAP can improve the overall cardiovascular prognosis of patients with SAHS by affecting more factors than simply the control of arterial hypertension; these include direct effects (more physiological modulation of daytime sympathetic and parasympathetic tone) and indirect effects (reduced incidence of arrhythmias) – provided that CPAP is administered in the long term.14,15
Khoo et al. emphasized that HRV parameters, and in particular the HF component, are highly sensitive to breathing patterns. We did not consider this parameter in our analysis, as we did not synchronize ECG monitoring by Holter with the polysomnography study.13 In our opinion, this would have prevented the correct analysis of changes in HRV during waking hours and the interpretation of the changes observed in HRV beyond the hours of sleep. Chrysostomakis et al. confirmed SAHS-induced changes in HRV, when they observed changes indicative of the nocturnal response to the parasympathetic stimulus that tended to normalize after starting CPAP.11 However, as we too found, this trend was not statistically significant in the analysis.
Impact of CPAP Therapy on the Incidence of ArrhythmiasParasympathetic nervous system dysfunction is well known to cause a higher incidence of cardiac arrthymias.3 We were able to show in our study that CPAP reduces the incidence of AT (P=.024) in a cohort of patients with a very little underlying heart disease burden, suggesting that this treatment may protect against the appearance of arrhythmias. While AT is a relatively benign arrhythmia, it sometimes needs to be controlled with antiarrhythmic agents, and it may also induce structural changes in the atrium that predispose to the development of AF. Thus AT does carry a certain degree of associated cardiovascular morbidity.25
In a previous paper, our group showed that CPAP therapy reduces the incidence of de novo AF in patients with atrial flutter.17 The possibility that CPAP reduces the incidence of AF and ventricular arrhythmias in a larger, more general population of SAHS patients requires further study.17–20
Data from patients with heart failure show that CPAP reduces the VE burden,20 but we could not confirm this in our study. The low prevalence of heart disease in our series may explain this discrepancy.
LimitationsThis study is not a randomized analysis of the effect of CPAP on HRV and the incidence of arrhythmias, and therefore cannot unequivocally prove the potential therapeutic benefit identified. However, the study design (analysis of paired data) minimizes the bias of interindividual variability and reduces this potential limitation. In view of our results, the possibility that changes in HRV and the incidence of arrhythmias after CPAP confer a better cardiovascular prognosis on SAHS patients appears reasonable, although it remains speculative.
The study design, in which the Holter monitoring was performed separately from the polysomnography analysis, prevented us from establishing a correlation between desaturation time and the incidence of arrhythmias or HRV. However, this same methodological aspect helped underline that the deleterious effect of SAHS (and the beneficial effect of CPAP) on HRV and incidence of arrhythmias goes beyond the periods of greatest desaturation.
Associated obesity-hypoventilation syndrome could not be ruled out in 8 of our patients with CT90%>30%, although correction of desaturations after CPAP (data not shown) appears to suggest that this change in CT90% was due to SAHS.
The limited sample size and the low prevalence of arrhythmias in our population may limit the analysis of the impact of CPAP on the incidence of arrhythmias, particularly with regard to ventricular episodes. Recruiting patients from the Respiratory Sleep Disorder Clinic obviated selection bias, but resulted in a series relatively free of heart disease and arrhythmias.
ConclusionsCPAP treatment is not associated with significant changes in HRV during sleep. The partial improvement in HRV and the incidence of atrial tachycardia episodes during waking hours may influence cardiovascular morbidity and mortality in patients with SAHS.
Authors ContributionsNuria Grau: study conception and design. Data collection.
Victor Bazan: study conception and design. Data collection and analysis. Manuscript preparation.
Mohamed Kallouchi: data collection.
Diego Rodríguez: study conception and design.
Cristina Estirado: data collection.
María Isabel Corral: data collection.
María Teresa Valls: data collection.
Pablo Ramos: critical review of the final manuscript.
Carles Sanjuas: study conception and design.
Miquel Felez: study conception and design.
Ermengol Valles: critical review of the final manuscript.
Begoña Benito: critical review of the final manuscript.
Joaquim Gea: study conception and design.
Jordi Bruguera-Cortada: critical review of the final manuscript.
Julio Marti-Almor: study conception and design. Critical review of the final manuscript.
Conflict of InterestThe authors declare that they have no conflict of interests.
We thank Sergi Mojal for his collaboration in performing the statistical analysis of this study.
Please cite this article as: Grau N, Bazan V, Kallouchi M, Rodriguez D, Estirado C, Corral MI, et al. Impacto a largo plazo del tratamiento con presión positiva continua en la vía aérea superior sobre la incidencia de arritmias y la variabilidad de frecuencia cardiaca en pacientes con apnea del sueño. Arch Bronconeumol. 2016;52:17–23.