Malignant pleural effusion (MPE) is common in clinical practice, affecting about 15% of patients with cancer in the course of their illness, typically indicating advanced neoplastic disease and a poor prognosis.1 It's paramount to stratify these patients and accurately predict survival, to allow individualized appropriated treatment. Several variables have been tested for the prediction of survival of MPE, namely pleural fluid pH,2 glucose,3 LDH3–5 and protein,4,6,7 serum neutrophil to lymphocyte ratio (NLR)6,7 serum albumin,8 histology of primary tumour,2,4,6,7 Eastern Cooperative Oncology Group performance status (ECOG PS)6,7,9 and Karnofsky index (KI),3,10 among others, with variable performances in prediction of survival across studies.
In 2014, Clive et al.11 created and validated the first score to predict the survival of MPE patients, the LENT score, which is calculated based on pleural fluid LDH (L), ECOG PS (E), serum NLR (N) and tumour type (T). Each of the prognostic indicators has a numeric value associated. Based on the calculated score, patients are stratified in risk groups: low (score 0–1), moderate (score 2–4) and high-risk (score 5–7), with median survival of 319, 130 and 44 days respectively, performing better than ECOG PS alone in the prediction of survival.
In 2018, Abisheganaden12 showed that LENT score underestimated survival in EGFR mutated lung adenocarcinoma (LA) patients and probably would need some adjustment in this subgroup.
We aimed at evaluating the performance of LENT score in predicting MPE survival in clinical practice and specifically in patients with MPE secondary to EGFR mutated LA.
We prospectively selected adult patients with a new and first diagnosis of MPE from January 2016 to December 2018 followed at our institution, regardless if the neoplasm was previously diagnosed or diagnosed simultaneously with MPE. Every pleural fluid sample sent for histopathologic examination was signalized. If the presence of malignant cells was confirmed the patient would be included in the study.
Besides LENT's variables, the following data was collected through consultation of medical records: sex, age at diagnosis of MPE, KI, timing of diagnosis of primary tumour, pleural effusion size and laterality, MPE time of diagnosis, pleural fluid differential cell count, pH, glucose, protein, treatment performed and time of death.
We used Kaplan–Meier with log-rank method to estimate survival in each group and to search for factors with impact in this outcome. All reported p values are two-tailed, with a p value of 0.05 indicating statistical significance. Statistical analysis was performed using the IBM SPSS Statistics for Windows, version 25.
We included 69 patients with a slight predominance of females (n=36; 52.2%) and a mean age of 70.9±12.8 years. Mean KI was 64.26±26.50% and mean ECOG PS was 1.84±1.31.
MPE was the first manifestation of malignancy in 73.9% of patients (n=51). Seventeen patients (24.6%) already had a previous diagnosis of cancer. In one patient it wasn’t possible to establish if MPE was secondary to a previous neoplasm or the newly diagnosed one.
The most frequent primary neoplasm observed was lung cancer (n=41; 59.4%), with LA accounting for the majority of cases (n=38; 92.7%), followed by hematologic malignancies (n=10; 14.5%), gastric cancer (n=4; 5.8%), breast cancer (n=3; 4.3%) and pleural mesothelioma (n=3; 4.3%).
All the pleural effusions were exudative and most showed a predominance of lymphocytes (n=58; 84.1%). Median pleural fluid LDH was 641.6 IQR (Interquartile Range) 366U/L, median protein was 42 IQR 11g/L and mean pH was 7.34±0.1.
Thirty-two patients (46.4%) showed a large effusion (occupation of more than 75% of the hemithorax) and there was a slight predominance of right-sided effusions (n=38; 55.1%).
In the majority of cases the diagnosis of MPE was made through cytology of pleural fluid (n=49; 71.0%), followed by pleural biopsy (n=15; 21.7%), medical thoracoscopy (n=4; 5.8%) and percutaneous transthoracic biopsy (n=1; 1.4%).
Most patients underwent treatment for MPE (n=62; 89.9%), 68.1% (n=47) with placement of chest tube, with 16 of these (23.2%) submitted to pleurodesis, and 21.7% (n=15) submitted to aspiration thoracentesis.
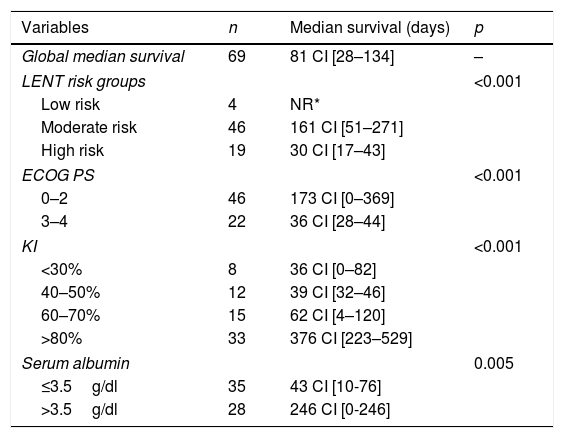
LENT score appropriately stratified patients according to their life expectancy (Table 1), with 75% of patients alive at 6 months in the low-risk group, 60.9% in the moderate-risk group and 5.3% in the high-risk group.
Global survival of MPE and survival according to LENT groups, ECOG PS, KI and serum albumin.a
| Variables | n | Median survival (days) | p |
|---|---|---|---|
| Global median survival | 69 | 81 CI [28–134] | – |
| LENT risk groups | <0.001 | ||
| Low risk | 4 | NR* | |
| Moderate risk | 46 | 161 CI [51–271] | |
| High risk | 19 | 30 CI [17–43] | |
| ECOG PS | <0.001 | ||
| 0–2 | 46 | 173 CI [0–369] | |
| 3–4 | 22 | 36 CI [28–44] | |
| KI | <0.001 | ||
| <30% | 8 | 36 CI [0–82] | |
| 40–50% | 12 | 39 CI [32–46] | |
| 60–70% | 15 | 62 CI [4–120] | |
| >80% | 33 | 376 CI [223–529] | |
| Serum albumin | 0.005 | ||
| ≤3.5g/dl | 35 | 43 CI [10-76] | |
| >3.5g/dl | 28 | 246 CI [0-246] | |
Concerning EGFR status: 8 of the 38 patients with LA carried the mutation, all of them classified as LENT moderate-risk. Nonetheless, they showed significantly longer survival (265 days CI [0–558] VS median not reached – mean of 506 days CI [343–668]; p=0.04) than patients with the same tumour and risk group without EGFR mutation.
We also found that patients with serum albumin of 3.5g/dl or lower and those with lower ECOG PS and higher KI had worse survival (Table 1).
In summary, MPE has a dismal prognosis and life expectancy should guide therapy, so that patients are offered treatment options that maximize the control of symptoms and quality of life in their last weeks or months.
Several variables influenced survival. Firstly the performance scores, ECOG PS and KI, matching other studies,3,6,9 an aspect already evaluated in LENT. Secondly, serum albumin, probably a surrogate for nutritional status, as it was also demonstrated in other publications8 and probably important to take into consideration when evaluating these patients, as it's not included in LENT.
Finally, LENT is a valuable tool in this setting but needs to be used with caution in MPE patients with LA carrying EGFR mutations, as it seems to underestimate their survival, even though these patients seem to have worse outcomes under tirosine kinase inhibitor therapy compared with patients with the same diagnosis but without MPE.13,14 Nevertheless, it seems clear they still have a better prognosis than LA patients with MPE with wild-type EGFR.
Although EGFR mutations appear only in a small subset of LA, this is frequently the most common malignancy causing MPE and, furthermore, there is data suggesting that the rate of EGFR mutation is higher in LA patients with MPE.15 These data highlight the importance of seeking tools to accurately predict survival in these patients.