Induced sputum is a non-invasive method for studying pulmonary inflammation.
ObjectivesTo assess pulmonary inflammation by analysis of induced sputum specimens in patients with systemic sclerosis and lung involvement, and to determine whether there is a correlation with the pulmonary function alterations in these patients.
MethodsTwenty-five patients with systemic sclerosis were included (20 women). Patients were divided into 3 groups according to the type of lung involvement: group 1, diffuse interstitial lung disease (n=10); group 2, those with pulmonary arterial hypertension (n=7), and group 3, patients with systemic sclerosis without lung involvement (n=8). All patients underwent a complete lung function study. Induced sputum samples were obtained and differential cell count was performed by optic microscopy.
ResultsThe mean percentage of sputum neutrophils was 85%, 71%, and 75% for groups 1, 2, and 3, respectively. A significant negative correlation between sputum total cell count and DLCO was seen in group 1 and group 3 (r=−0.733, P=.016; and r=−0.893, P=.007, respectively). This negative correlation was not observed in group 2.
ConclusionsPulmonary inflammation was present in all patients with systemic sclerosis included in the study, regardless of the presence of documented signs of pulmonary involvement. This finding suggests that induced sputum could be helpful for detecting early abnormalities indicative of subclinical pulmonary involvement in patients with systemic sclerosis.
El esputo inducido es un método no invasivo para estudiar la inflamación pulmonar.
ObjetivosEstudiar la inflamación pulmonar mediante el análisis de muestras de esputo inducido en pacientes con esclerosis sistémica y afección pulmonar, y determinar si existe correlación con las alteraciones de la función pulmonar observadas en estos pacientes.
MétodosSe incluyeron 25 pacientes con esclerosis sistémica (20 mujeres). Los pacientes fueron clasificados en 3 grupos, considerando el tipo de afección pulmonar: grupo 1, enfermedad pulmonar intersticial difusa (n=10); grupo 2, hipertensión arterial pulmonar (n=7), y grupo 3, pacientes con esclerosis sistémica sin afección pulmonar (n=8). A todos los pacientes se les realizó un estudio completo de función pulmonar y se obtuvieron muestras de esputo inducido. El recuento celular diferencial en las muestras de esputo se realizó mediante microscopia óptica.
ResultadosEl porcentaje medio de neutrófilos en esputo inducido fue del 85, del 71 y del 75% para los grupos 1, 2 y 3, respectivamente. Se observó una correlación negativa significativa entre el recuento celular total en esputo inducido y la DLCO en los grupos 1 y 3 (r=−0,733, p=0,016; y r=−0,893, p=0,007, respectivamente). Esta correlación negativa no se observó en el grupo 2.
ConclusionesEn todos los pacientes con esclerosis sistémica incluidos en el estudio se detectó inflamación pulmonar, a pesar de la presencia o no de signos documentados de afección pulmonar. Este hallazgo sugiere que el esputo inducido podría ser una técnica útil para detectar anomalías tempranas indicativas de afección pulmonar subclínica en pacientes con esclerosis sistémica.
The prevalence of pulmonary fibrosis in systemic sclerosis (SSc) varies between 25% and 90%, depending on the method used for detecting diffuse interstitial lung disease (DILD).1 Pulmonary fibrosis is associated with higher mortality in patients with SSc2–6 and it is currently the main cause of death in this population.7 Therefore, precise, early diagnosis of pulmonary affectation is necessary to avoid the progression toward fibrosis.
The first manifestation of the pulmonary fibrotic process that takes place in SSc is alveolitis. This fibrosing alveolitis can be detected by means of lung function tests and high-resolution computed tomography (HRCT).8–10 Lung function evaluation is the only widely available technique for determining whether the disease is severe enough to justify immediate therapeutic intervention. HRCT is the most sensitive and specific tool to identify pulmonary fibrosis in SSc, but the results are not necessarily indicative of clinically relevant DILD.8,9,11
The bronchoalveolar lavage (BAL) samples of patients with SSc and DILD show high granulocyte counts, specifically neutrophils and eosinophils,10,12,13 but there is no sufficient evidence for recommending BAL in order to evaluate the activity of the disease or the probability of response to treatment.14 In addition, BAL is an invasive diagnostic technique, which makes it of little use as a follow-up method of lung inflammation.
Induced sputum (IS) is a non-invasive diagnostic technique used to study cell types and different inflammatory markers in the respiratory tract.15 This method has been widely used to evaluate pulmonary inflammation in asthma16–18 and in COPD.18 It has been demonstrated that IS can also be used to study DILD, specifically sarcoidosis19 or Sjögren's syndrome.20 However, according to our knowledge, in the literature there are very few studies that evaluate the potential use of IS for the study of lung affectation associated with SSc.21,22 The pathogenesis of SSc includes an inflammatory activation that is thought to play a significant role in the disease. In addition, the demonstration of this inflammatory process could be important for diagnosis, treatment and progression of this entity.10
The objective of this present study was to determine the inflammatory cell profile in the IS of patients with SSc, evaluate the clinical usefulness of the findings obtained and analyze whether the results of the IS analysis correlate with the lung function parameters in these patients.
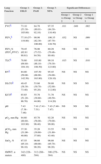
Materials and MethodsStudy PopulationOur is a cross-sectional study that included 25 Caucasian patients with SSc (20 women) who were consecutively seen in the Scleroderma Unit of the Internal Medicine Department at our hospital between 2006 and 2009, with compatible immunology.23 The study was approved by the Ethics Committee for Clinical Research of the hospital, and all the patients signed a consent form to participate in the study. Mean age was 58.52±14.80 (range: 24–82 years) and the mean duration of the disease, calculated from the onset of the first Raynaud's symptoms, was 20.85±14.40 years (range: 0.7–57). Twenty-one patients had limited SSc and 4 diffuse SSc. All the patients had positive antinuclear antibodies (ANA), 14 patients also presented positive anti-centromere antibodies (ACA) and 3 had positive anti-Scl-70 antibodies. At the moment of inclusion, 4 patients were being treated with immunosuppressant drugs (1 azathioprine and 3 sodium mycophenolate) and 3 were treated with bosentan (Table 1). The exclusion criteria for participation in the study were the presence of other inflammatory respiratory diseases (such as asthma, COPD and sarcoidosis) and/or infection of the respiratory tract present during the month previous to obtaining the IS sample.
Demographic Variables and Baseline Characteristics of the Patients Included in the Study.
| Patients | Total25 | Group 1: DILD10 | Group 2: PAH7 | Group 3: NPA8 |
| Sex, F:M | 20:5 | 7:3 | 6:1 | 7:1 |
| Age, yearsb | 58.52 (14.80) [24–82] | 56.60 (19.82) [24–82] | 60.86 (6.34) [54–71] | 58.88 (14.16) [31–75] |
| Smoker/non-smoker | 2/23 | 0/10 | 1/6 | 1/7 |
| Disease onset, SScl:SScd | 21:4 | 7:3 | 7:0 | 7:1 |
| Disease duration, yearsb | 20.85 (14.40) [0.7–57] | 21.47 (16.85) [0.7–57] | 19.56 (10.83) [1.2–34.5] | 20.86 (15.62) [2–48.5] |
| Treatmenta | 7 (28) | 5 (50) | 2 (29) | 0 (0) |
| ANAa | 25 (100) | 10 (100) | 7 (100) | 8 (100) |
| ACAa | 14 (56) | 3 (30) | 6 (86) | 5 (63) |
| Scl-70a | 3 (12) | 3 (30) | 0 (0) | 0 (0) |
| Ground glassa | 9 (36) | 8 (80) | 1 (14) | 0 (0) |
| Reticular patterna | 6 (24) | 6 (60) | 0 (0) | 0 (0) |
ACA, anti-centromere antibodies; ANA, antinuclear antibodies; DILD, diffuse interstitial lung disease; SScd, diffuse systemic sclerosis; SScl, limited systemic sclerosis; PAH, pulmonary arterial hypertension; NPA, no pulmonary affectation.
The study population was divided into 3 groups based on the lung affectation: group 1 included patients with DILD (n=10); group 2, patients with pulmonary artery hypertension (PAH) (n=7); and group 3, patients with no pulmonary affectation (NPA) (n=8). The presence of DILD and PAH was established prior to performing the study. DILD was defined in accordance with the following criteria: a restrictive respiratory pattern in the lung function tests and/or radiological evidence of interstitial disease on chest radiography or HRCT. PAH was determined by Doppler ultrasound (systolic PAP >40mmHg)23 or with cardiac catheter (mean PAP >25mmHg).
All of the patients included in the study underwent complete lung function studies, arterial blood gas analysis, 6-min walk test (6MWT) and induced sputum.
Lung Function TestsThe lung function tests were done using MasterLab equipment (MasterLab, Jaegger, Germany) and following the recommendations of the European and American Respiratory Societies (ERS and ATS).24,25 The static pulmonary volumes were registered by plethysmography,25 and DLCO (carbon monoxide diffusing capacity) was measured using the single-breath method.26 For the analysis of the spirometry, the reference values proposed for Mediterranean populations were used27; the values used for the static lung volumes and DLCO were those established by the ERS and ATS.25
The restrictive ventilatory pattern was defined as a total lung capacity (TLC) <80% predicted.28 The obstructive ventilatory pattern was defined based on the forced expiratory volume in 1s (FEV1)/forced vital capacity (FVC) ratio <70%, together with FEV1 <80% predicted.28 The concomitant presence of characteristic functional criteria for both patterns was defined as a mixed ventilatory pattern. DLCO was considered low if it presented levels <80% predicted.28
Blood GasesThe sample for arterial blood gas studies was obtained in accordance with the recommendations of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR).29 After the administration of local anesthesia, the radial artery was punctured with a heparinized syringe. The samples were processed immediately using a co-oximeter IL 682 (Instrumentation Laboratories, Lexington, MA, USA).
6-Min Walk TestThe 6MWT was done following the ATS recommendations.30 For the analysis of the results, desaturation and the total distance walked in meters during the 6MWT were registered.
Obtaining and Processing the Induced Sputum SamplesThe induction and the processing of the sputum were done following the method described by Pizzichini et al.,15 making the patient inhale increasing concentrations of hypertonic saline solution (3%, 4%, and 5%). The sputum samples were analyzed within 2h after having been obtained.15
For the processing of the of the sputum samples, we selected mucus plugs, which were deposited in an Eppendorf tube and weighed. The sample was treated with a volume of solution of DTT (dithiothreitol) equivalent to 4 times for every mg of sputum obtained; it was agitated during 10min for homogenization. Later, PBS was added at a volume equivalent to 4 times each mg of sputum, and the sample was agitated for 5min more. A small portion (15μl) was used for the total leucocytes count (in a Neubauer chamber) and to determine the cell viability with the trypan blue exclusion method. The rest of the sample was centrifuged and we opted to separate the supernatant from the cellular sediment. With the sediment, we obtained preparations that were stained with May–Grünwald–Giemsa for the differential count, counting 500 non-squamous cells and expressing the result as a percentage of the non-squamous cell total present in the samples. In all the sputum samples, we determined the quantitative cellular content of total cells expressed as cells/ml.
Statistical AnalysisThe differences between the groups studied were analyzed using the Kruskal–Wallis test. A P value <.05 was considered significant. In order to evaluate correlations between the cell subtypes in IS and the lung function parameters, Spearman's correlation coefficient was used. In order to carry out the different statistical analyses, the SPSS program (version 13.0) for Windows (SPSS Inc., Chicago, IL) was used.
ResultsDemographic Data and Baseline CharacteristicsTable 1 shows the demographic data and the baseline characteristics of the study population.
Lung Function StudiesTable 2 summarizes the lung function data of the patients studied. Significant differences were found in the FVC values among the three study groups. The FEV1 values were also significantly different between group 1 and groups 2 and 3 (P=.032 and P=.009, respectively). In addition, significant differences were obtained in the TLC values between group 1 and groups 2 and 3 (P=.015 and P=.010, respectively).
Values of the Lung Function Parameters Studied.a
| Lung Function | Group 1: DILD | Group 2: PAH | Group 3: NPA | Significant Differences | ||
| Group 1 vs Group 2 | Group 2 vs Group 3 | Group 1 vs Group 3 | ||||
| FVCb | 73.10 (53.30–105.60) | 84.70 (80.01–92.10) | 97.35 (84.30–116.40) | .011 | .049 | .002 |
| FEV1b | 77.10 (57–119.80) | 90.90 (82.30–100.00) | 106.35 (87.70–128.70) | .032 | NS | .009 |
| FEV1% (FEV1/FVC) | 79.45 (62.81 90.61) | 76.90 (73.52–84.56) | 80.29 (70.46–89.46) | NS | NS | NS |
| TLCb | 78.60 (69.80–104.10) | 103.00 (86.10–108.50) | 99.10 (79.30–123.60) | .015 | NS | .010 |
| RVb | 84.80 (59.00–142.50) | 107.50 (68.90–163.90) | 95.45 (54.00–128.10) | NS | NS | NS |
| DLCOb | 49.45 (38.30–73.40) | 53.60 (30.70–95.20) | 58.80 (52.00–112.00) | NS | NS | NS |
| KCOb | 65.65 (51.20–86.70) | 55.70 (30.80–44.00) | 71.70 (49.50–114.20) | NS | NS | NS |
| pH | 7.43 (7.38–7.46) | 7.41 (7.41–7.51) | 7.43 (7.40–7.47) | NS | NS | NS |
| pO2, mmHg | 94.60 (80.80–103.90) | 85.70 (58.00–96.40) | 92.20 (79.00–99.00) | NS | NS | NS |
| pCO2, mmHg | 37.30 (31.00–40.00) | 35.20 (30.60–37.60) | 33.55 (31.60–43.40) | NS | NS | NS |
| SaO2, % | 97.55 (95.10–98.10) | 96.60 (90.00–98.20) | 96.80 (95.70–98.20) | NS | NS | NS |
| 6MWT, n meters | 342 (228–480) | 312 (288–504) | 306 (276–384) | NS | NS | NS |
DLCO, carbon monoxide diffusing capacity; DILD, diffuse interstitial lung disease; FEV1, forced expiratory volume in 1s; FEV1%, FEV1/FVC ratio expressed as percentage; FVC, forced vital capacity; PAH, pulmonary arterial hypertension; KCO, DLCO/alveolar volume; NS: not significant; 6MWT: 6-min walk test; RV: residual volume; NPA, no pulmonary affectation; TLC: total lung capacity.
Eight patients from group 1 showed ventilatory alterations: 7 patients presented restrictive ventilatory disorder and 1 patient showed an obstructive disorder. In the remaining 17 patients (2 from group 1 and all the patients from groups 2 and 3), spirometry and the plethysmography results were normal. The DLCO values were less than 80% of the predicted value in all the patients except in 3 (1 from group 2, and 2 from group 3), who presented normal values. The total distance walked during the 6MWT was <300m in 3 patients of group 1, 2 patients from group 2 and 3 patients from group 3. In these last three patients, the lower values found in the 6MWT were due to osteomuscular affectation. PO2 was <80mmHg in 1 patient from group 2 and in one patient from group 3.
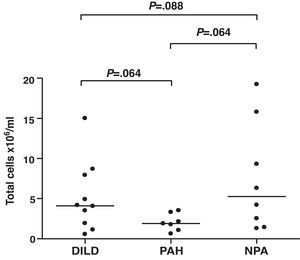
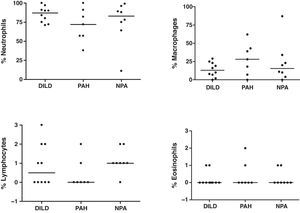
Induced SputumIn all patients, the induction of sputum was well-tolerated and no adverse effects were observed. The samples analyzed presented an average percentage of viability (range) of 77% (60–96), 81% (73–87), and 80% (64–94) for groups 1, 2 and 3, respectively. The total and differential counts (%) in the IS of the patients studied are shown in Figs. 1 and 2, respectively. Differences were found in the total cell count between the three groups studied, although they did not reach statistical significance (Fig. 1). A negative correlation was found between the total number of cells in IS and DLCO in the patients with DILD and NPA (r=−0.733, P=.016; and r=−0.893, P=.007, respectively). This negative correlation was not observed in the group of patients with PAH. The mean percentages of neutrophils in IS were 85%, 71%, and 75% for groups 1, 2, and 3, respectively (Fig. 2). No significant differences were found in the differential count among the three groups studied.
DiscussionThe present study evaluates the cellular profile and the clinical utility of the analysis of IS in patients with SSc, with and without lung affectation. Likewise, it researches whether the findings obtained in the analysis of IS correlate with the pulmonary alterations found in this pathology. The most relevant observation of the study is the high percentage of neutrophils found in the sputum of patients with SSc, regardless of whether they have pulmonary affectation or not, and the negative correlation between the total number of cells in IS and DLCO in patients with DILD and NPA.
BAL has been used for the early diagnosis of lung affectation in SSc. However, it is an invasive technique that is not adequate for follow-up studies over the course of the pathology. One alternative is the cytologic analyses of IS, as it is a non-invasive method that can detect pulmonary inflammation.31,32 Nevertheless, in spite of the fact that different criteria have been used to define the BAL pattern in SSc, including the presence of eosinophilia, neutrophilia and/or lymphocytosis,33 these criteria have not been defined for IS samples.
In the present study, a high percentage of neutrophils has been found in patients with SSc compared with healthy individuals.34,35 These results are similar to those obtained in two recent studies by Damjanov et al.21 and Vatrella et al.,22 who observed a significant increase of neutrophils and a low percentage of macrophages in the IS of patients with SSc and with lung affectation. In the study by Yilmaz et al.,36 however, the authors find a high percentage of macrophages and lymphocytes in the IS and a reduction in the percentage of neutrophils in patients with SSc. Likewise, these authors find that there is a correlation between the cell profile in IS and BAL in the patients studied. In this sense, there is a disparity between the data obtained in different studies, and Damjanov et al.21 and Fireman et al.32 observe that the cell profile in BAL does not correlate with that obtained in IS in the patients with SSc, concluding that it is not possible to make comparisons between both types of samples. This disparity between the data obtained in different studies could be due to the fact that BAL provides information about the distal airway and the alveolar spaces, which are rich in macrophages, while the IS is indicative of the upper and middle airways, presenting abundant neutrophils.21,32
In the present study, the high percentage of neutrophils found in the group of patients that did not present evidence of lung affectation could suggest the hypothesis that perhaps there is a sub-clinical pulmonary inflammatory process in SSc. In fact, there is evidence that, indeed, in patients with SSc there is subclinical inflammation. Along these lines, Ferri et al.37 found characteristics of DILD in autopsies of SSc patients. Nevertheless, clinically significant DILD occurs in less than 50% of patients with SSc. In addition, several authors have observed that studies in BAL generally present anomalies in patients with SSc without lung affectation.12,14 The clinical significance of this latent subclinical inflammation, both in BAL as well as in IS, is not known. With regards to the evolution of the cellularity in BAL, recent studies have demonstrated that it does not predict the subsequent evolution of the disease, nor the response to the immunosuppressant treatment.10,33 Without a doubt, the pulmonary inflammation is what initiates and maintains the fibrotic process; therefore, the treatment with immunosuppressants is indicated in patients with DILD.14 However, both the decision to initiate treatment as well as the evaluation of the response to it is not done depending on the inflammation detected in the BAL, but instead on the respiratory function repercussions and/or the extension of DILD detected in HRCT. Thus, some authors have suggested that, in patients with latent inflammation, periodical spirometries and radiological exams should be done and immunosuppressant therapy should be considered when alterations are observed in these parameters.10
In addition to the latent inflammation found in the IS samples of these patients, the majority of them (88%) presented an early alteration in DLCO. Despite the fact that the patients with DILD and PAH present a decline in DLCO, this should be less frequent in patients with SSc without pulmonary affectation.38,39 According to several authors, it is possible to see decreases in DLCO in 2%–40% of patients without lung alteration,40–42 although the significance of this decrease is also debated regarding its use for predicting the evolution of the disease toward DILD or PAH. In the largest series published to date, which includes 152 patients with diffusion alterations and without lung affectation, 27% of the cases develop mild DILD during follow-up, with FVC values between 70% and 80% predicted, and 7% develop PAH.38 In this present series, a lower diffusion was observed in all the patients with DILD, in all the patients with PAH except in one, and in 6 of the 8 patients in the NPA group. In this latter group of patients, there are several factors that could explain this decrease in DLCO. Tobacco is a potential cause, but only one of the patients with SSc and NPA was a smoker. Anemia may also contribute to the decline in DLCO,26 but none of the patients in this group showed abnormal hemoglobin values. Although it is much less probable, we cannot exclude a possible increase in endogenous CO, which has been observed in conditions that implicate a higher metabolism, such as in SSc, or in pulmonary microcirculation alterations, also present in this pathology.43 Considering the latent lung inflammation observed in the group of patients without lung affectation, it is possible that the decline in DLCO is related with this factor. The inverse correlation found between the number of total cells in the IS samples and the DLCO values support this idea. This hypothesis is reinforced by the fact that this correlation was also found in the group of patients with DILD, but not in the patients with PAH, who also showed less (although not significantly) total cells (Fig. 1). Some authors have suggested that a decline in diffusion below 50% predicted or an FVC/DLCO ratio greater than 1.4 could indicate progression toward PAH and a poor prognosis.38,39 In the present study, none of the patients in the NPA group had a decrease in diffusion below 50% predicted. This fact, together with the cell levels in IS, could suggest that the patients with low DLCO and an increase in neutrophils in IS present a greater risk for progression toward DILD rather than toward vascular affectation.
The study presents some limitations, such as the limited number of patients, which make future studies necessary with larger sample sizes in order to confirm these hypotheses. In addition, there is no control group. However, several studies have researched the normal cell levels in IS, therefore these results can be used to make comparisons,35,44 as has been done in this present study. Another limitation is not having a follow-up of the patients with SSc without pulmonary affectation, which makes the relationship between the increase of neutrophils in IS and the decrease in DLCO questionable.
In conclusion, in the present study we have observed lung inflammation in all the patients with SSc, regardless of whether there was a presence of pulmonary affectation or not. This finding suggests that IS could be useful for the early detection of a subclinical pulmonary affectation in patients with SSc. In addition, the analysis of IS could be useful as a tool for differentiating between SSc patients with low levels of DLCO and neutrophilic inflammation in IS whose progress could be toward DILD, and those with a normal differential cell count in IS whose progression could be toward PAH. Future follow-up studies are necessary with a greater number of patients in order to confirm the findings of the present study and to more precisely define the role of IS in this pathology.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Sánchez-Vidaurre S, et al. Inflamación pulmonar latente en pacientes con esclerosis sistémica. Arch Bronconeumol. 2011;48:8-13.