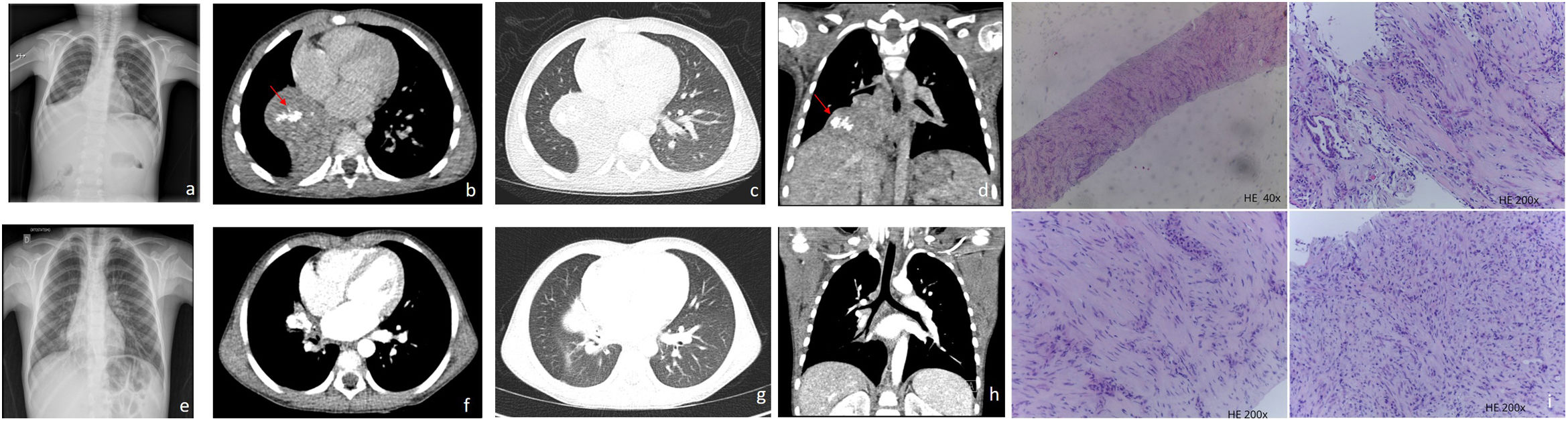
A five-year-old boy with a history of recurrent right lower lobe pneumonia presented in the emergency department with fever, dry cough and diminished vesicular murmur in the right lung. Blood tests revealed leucocytosis with neutrophilia. Serologic and microbiologic tests were negative. A chest radiography (Fig. 1a) showed a hypotransparency in the right lower lobe (RLL). Chest-CT showed (Fig. 1b–d) a round mass with gross calcifications in the RLL which caused atelectasis, as a result of obstruction of the intermedius bronchus.
(a) Chest radiograph shows a lung opacity projected in the right lower lung caused by atelectasis of the middle and right lower lobe. (b)–(d) CT post contrast images show a right paramediastinic smooth contour heterogeneous enhancing mass with calcifications (red arrows) measuring 47mm×47mm causing post obstructive atelectasis of the middle and right lower lobe. (e) Chest radiograph one-year pos chemotherapy shows a lung nodule in the right hemithorax adjacent to the right atrium. (f)–(h) CT post contrast images one-year post chemotherapy shows important reduction of the mass size, which is confined to the right hilum. (i) Core needle of a lung inflammatory myofibroblastic tumor is characterized by a proliferation of spindle fibroblastic–myofibroblastic cells with variation in cellularity and stroma and focal inflammatory infiltrate. In the upper right picture, a small lung alveolar space is seen.
A CT-guided transthoracic biopsy was performed and histology suggested an inflammatory myofibroblastic tumor (IMT) (Fig. 1i). Additional molecular characterization by NGS, revealed a TFG(4)-ROS1 rearrangement.
Given the encasement of the bronchus and the inferior pulmonary vein, the tumor was considered unresectable. Based on the molecular profile, crizotinib therapy was started with the aim of performing surgery at maximum tumor shrinkage with complete resection. The clinical condition rapidly improved. There has been observed toxicity. Chest-CT performed regularly, shows a continuously downsizing of the tumor (Fig. 1e–h).
IMT is a rare mesenquimal neoplasm which has a tendency for local recurrence (25%) but rarely metastasize. It can occur at any age but tends to affect children and young adults.1,2
It frequently appears as a solitary lesion and may develop throughout the body, mostly in the lung or in the abdomen.1
The symptoms vary according to the site of tumor origin.1,2 Pulmonary IMTs in children manifest with cough, fever, chest pain and hemoptysis.2
On imaging, IMT manifest as a heterogeneous well-defined mass, often with calcifications and a predilection for the periphery of the lower lobes,1,2 such as in our case. Prominent enhancement and nonenhancing central areas due to necrosis are typical. Pleural effusion and atelectasis are frequently present. Invasion of adjacent structures occurs in aggressive tumors.2
The imaging differential diagnosis includes pulmonary sequestration and type 2 or 3 pleuropulmonary blastoma. Local invasion may suggest metastasis, lymphoma or inflammatory fibrosarcoma.4
Surgical excision is the treatment of choice.1–3 Traditionally, for unresectable disease, chemotherapy with vinblastine and methotrexate were used, however due to their efficacy, targeted inhibitor-based therapy is currently the standard of care.3
Anaplastic lymphoma kinase, a receptor-type protein tyrosine kinase, is present in 50% of these tumors, but other emerging genetic alterations in ALK-negative IMT (ROS1 NTRK, RET, PDGFR beta) which are targetable biomarkers, could help to improve the management of patients with ALK-negative disease.3
Following ALK-fusions, ROS-1 related fusions are the most prevalent alterations in pediatric lung IMT. Tyrosine kinase inhibitors such as crizotinib are equally successful in tumors driven by either ALK or ROS1 oncogenic fusions.4,5 Larger studies are needed to better define the duration of treatment.
A close follow-up with cross sectional imaging is important for the early recognition of tumor progression.3
Primary pulmonary tumors are rare in childhood with IMT being the most common.1 Knowledge of this entity is crucial in the presence of recurrent respiratory symptoms accompanied by a lung opacity in the radiography.
Availability of data and materialsThe data are available from the author upon reasonable request.
Consent for publicationWritten informed consent was obtained from the patient for the publication of this case report. The data presented in this case report do not allow identification of the patient.
FundingNo funding was obtained.
Conflict of interestThe authors declare that there is no conflict of interest.
A special thank you note to the pathologist MD. Helena Barroca for providing the histology images.