Endocan levels were found to be associated with severity and mortality of the respiratory system diseases.
ObjectiveWe aimed to figure out whether endocan was an important marker for the diagnosis, severity and follow-up of bronchopulmonary dysplasia (BPD).
Materials and methodsInfants with moderate/severe BPD, and who required hydrocortisone treatment were included in the study group. Infants without BPD were allocated in the control group. Endocan levels were compared between the control group and the study group, and before and after the treatment in the study group.
ResultsA total of 148 infants, 74 infants in the control group and 74 infants in the BPD group, were included. The endocan level was higher in the BPD group than in the control group (P = .001). Endocan levels before treatment in the BPD group was found to be higher than endocan level after treatment (P = .021).
ConclusionOur study found that endocan levels increased in moderate/severe BPD. Serum endocan levels may be a safe and novel indicator for the follow-up of response to treatment and the prognosis of the severity of the disease.
Los niveles de endocan se han asociado con la mortalidad y la gravedad de enfermedades del aparato respiratorio.
ObjetivoEl objetivo fue averiguar si el endocan es un marcador útil para el diagnóstico, la gravedad y el seguimiento de la displasia broncopulmonar (DBP).
Materiales y métodosSe incluyeron en el estudio lactantes con DBP moderada/grave que requirieron tratamiento con hidrocortisona (grupo DBP). El grupo control lo constituyeron lactantes sin DBP. Los niveles de endocan se compararon entre el grupo control y el grupo de estudio y, en este último, tanto antes como después del tratamiento.
ResultadosSe incluyeron un total de 148 lactantes; 74 en el grupo control y 74 en el grupo DBP. Los niveles de endocan fueron más elevados en el grupo DBP que en el grupo control (p=0,001). Los niveles de endocan también resultaron superiores en el grupo DBP antes del tratamiento que después del mismo (p=0,021).
ConclusiónNuestro estudio halló que los niveles de endocan se encuentran incrementados en la DBP moderada/grave. Los niveles séricos de endocan podrían utilizarse como un nuevo indicador seguro para el seguimiento de la respuesta al tratamiento y el pronóstico de gravedad de la enfermedad.
Bronchopulmonary dysplasia (BPD) is a common pulmonary morbidity occurring due to multifactorial causes such as prematurity, mechanical ventilation therapy, oxygen toxicity in preterm infants with respiratory distress syndrome.1–4 Considering the decisions taken at The National Institute of Health consensus conference, it can be divided in to three different categories as mild, moderate, or severe in accordance with the period of respiratory support at 28 days of postnatal age and 36 weeks of postmenstrual age (PMA).5 While some cellular and humoral factors are both effective in pathogenesis and can serve as biomarkers for following the disease, others may have assigned as biomarkers of BPD. The presence of novel biomarkers characterizing disease activity and severity in early period would be useful in the follow-up of the disease processes and response to treatment. Therefore, early initiation of appropriate therapy for BPD will provide better lung development in these infants who have improperly arrested lung development in the early stages.2
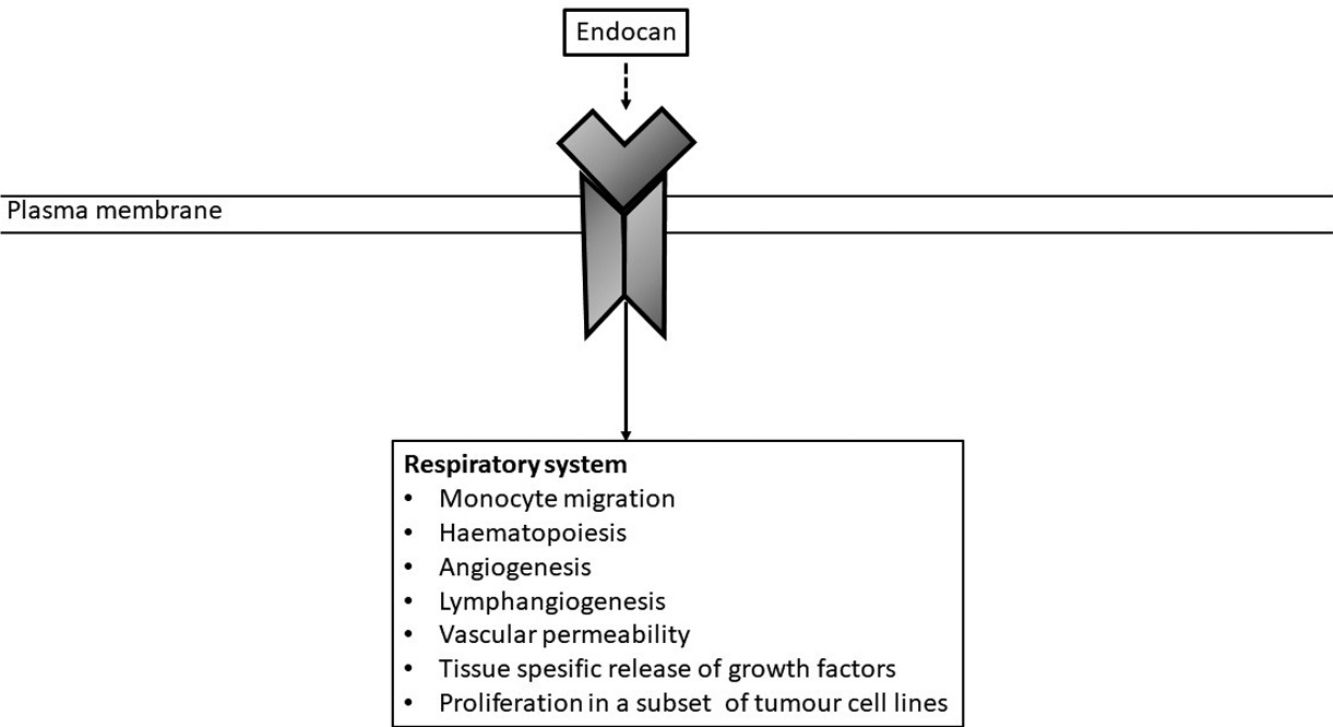
Endocan, a new discovered proteoglycan and previously termed as endothelial cell specific molecule 1 (ESM-1), has been reported to be expressed mainly by renal and pulmonary endothelium.6 Although it is known to be released from the lung endothelium, its precise role is still unclear. However, Endocan is regarded as an encouraging agent to figure out and predict the different inflammatory conditions and malignant diseases of the lung.6 Some markers that mediate the pathogenesis of BPD have been investigated, and evidence has emphasized the association between the severity of BPD and these markers.2 However, there are no data about the relationship between severity of BPD and serum Endocan levels. We therefore aimed to ascertain the association between the severity of BPD and serum endocan levels in very low birth weight (VLBW) preterm infants. Additionally, the second aim of our study was to investigate the utility of endocan levels in evaluating response to hydrocortisone (HC) treatment in infants with BPD.
Materials and methodsStudy design and patient selectionOur study was a prospective nested case–control study conducted in the neonatal intensive care unit (NICU), between November 2014 and June 2017. The local ethical committee was approved the study protocol, and all parents signed the informed consent before the study.
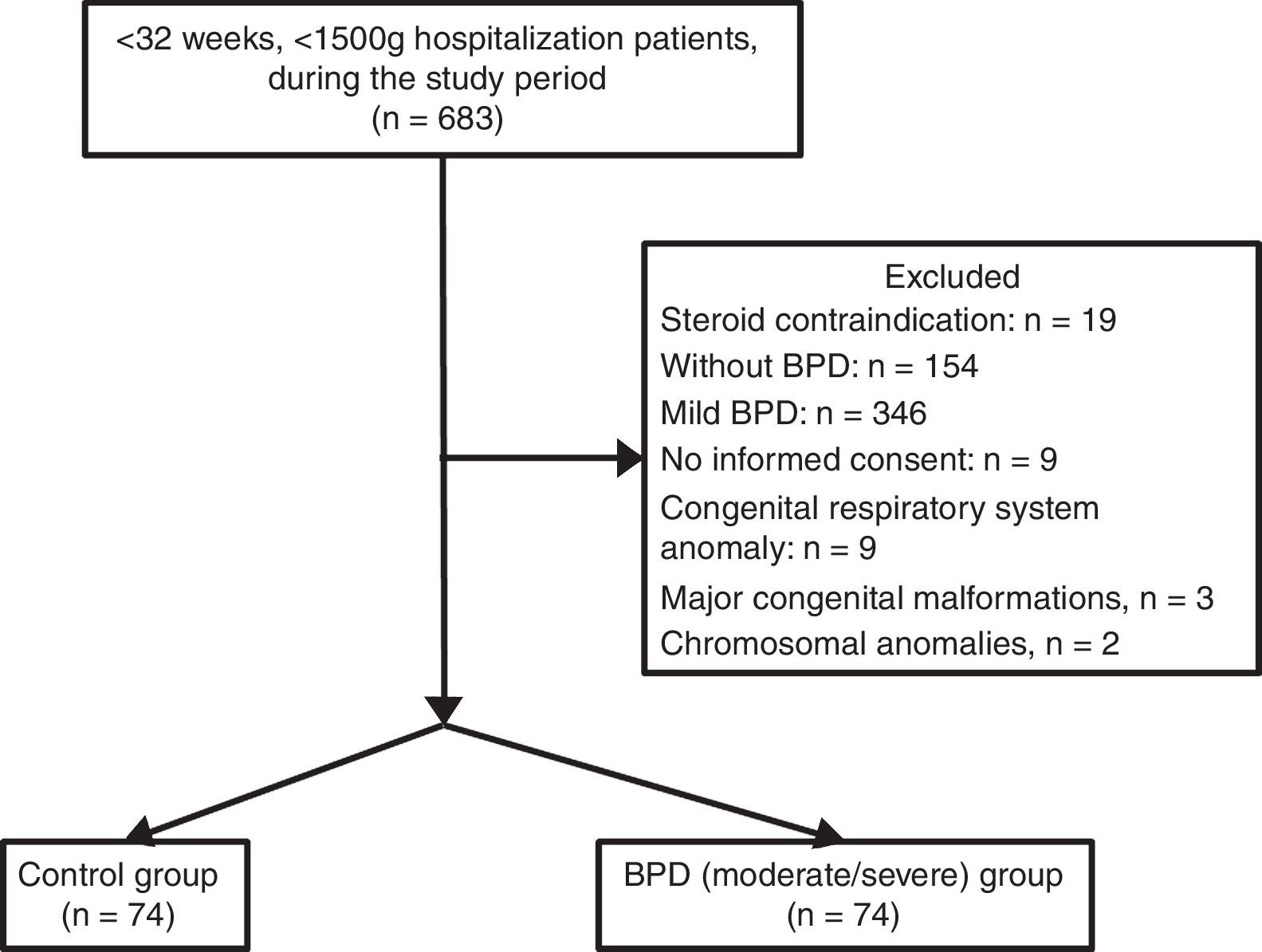
During the study period, postpartum cord blood samples were obtained from all infants with gestational age of <32 weeks and birth weight of <1500g. Subsequently, separated serum samples were stored at −80°C until measurement. All preterm infants were cared and followed in NICU. All infants whose gestational age <32 weeks and VLBW were included in the study. Infants whose parents did not give permission to be included were excluded from the study. Infants with congenital respiratory system anomalies, chromosomal anomalies, major congenital malformations, infants with pulmonary hypertension, hypotension with or without heart failure requiring inotropic drugs support, pneumonia, sepsis, mild BPD, and infants without BPD were excluded. Infants with BPD (moderate/severe) were assigned as the study group. Additionally, infants with BPD were divided in to two groups according to hydrocortisone treatment at PMA 36 weeks; as treated with hydrocortisone and without hydrocortisone. Premature infants without respiratory support with either invasive or non-invasive mechanical ventilation, infants with fully enteral fed and clinically stable were allocated as the control group at PMA 36 weeks.
Neonatal intensive care unit protocols were applied to all patients for general supportive care during their stay. Demographic and clinical characteristics of the infants involving: gender, birth weight (BW), gestational age (GA), mode of delivery, Apgar score at 1st min and 5th min, antenatal steroid administration, duration of non-invasive ventilation and mechanical ventilation, duration of oxygen support, respiratory distress syndrome (RDS), patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH; grade ≥3), necrotizing enterocolitis (NEC; grade ≥2), retinopathy of prematurity (ROP) requiring laser treatment, and length of NICU stay were recorded.
Infants with RDS were recognized requirement of surfactant administration for any situation.7 Retinopathy of prematurity was determined by skilled ophthalmologists based on the international classification of retinopathy of prematurity.8 Intraventricular hemorrhage was identified by cranial ultrasonography examination in the first 7 days of life.9 Bell's criteria were considered to identify NEC.10 According to consecutive echocardiographic examinations and clinic criteria, infants with PDA were treated with either certain drugs (ibuprofen and/or paracetamol)/or surgical ligation.11
Defining bronchopulmonary dysplasiaInfants with moderate or severe BPD were included in the study group. BPD was defined according to oxygen requirement or positive pressure ventilation therapy for a certain period of time (days) (e.g., at postmenstrual age 36 weeks or over postnatal day 28). Mild BPD was considered for infants who had oxygen requirement throughout the first 28 days but not at 36 weeks of PMA or at discharge. Moderate BPD was identified as oxygen requirements over 28 days of postnatal life plus oxygen supplementation with less than 30% oxygen level at 36 weeks of PMA. Severe BPD was defined as oxygen-dependence with 30% oxygen or greater over the first 28 days of postnatal life and/or positive pressure support at 36 weeks of PMA.12
Hydrocortisone treatment protocol for bronchopulmonary dysplasiaHydrocortisone treatment was given to infants with moderate and severe BPD. Therefore, eligible infants with moderate or severe BPD at postmenstrual age of 36 weeks were treated with HC and included in the study.1 Standard clinical dosage schemes for HC were fulfilled. Dosages of HC with 5mg/kg/d separated in to 4 different doses for 7 days and following a subsequent decreasing dose were applied for each 5 days. A total cumulative dosage of 72.5mg/kg and a period of 22 days for a standard therapy were completed for every selected infant.13 Infants who had contraindications for hydrocortisone treatment [osteopenia (alkaline phosphatase levels >700U/L), hyperglycemia (serum glucose levels >200mg/dL, hypertension (systolic and diastolic blood pressure above 95% percentile according to postnatal age), infection (meningitis, sepsis, pneumonia and urinary tract infection) and cardiac hypertrophy (detected by echocardiography)] were not treated with hydrocortisone.
Blood samplingBlood samples were obtained from infants who were diagnosed as moderate or severe BPD at 36 weeks of PMA. And also blood samples for the measurement of Endocan were obtained before starting and after hydrocortisone cure. The levels of Endocan were evaluated in the control group at 36 weeks of PMA. Simple glass tubes covering no separation gel or preservatives were used for the collection of blood samples. Clotting of blood was waited for a while (30minutes), and subsequently centrifuged at 3000rpm at 4°C for 10min. 1mL of serum aliquots were separated and immediately frozen at −80°C until evaluation. Before starting measurement, serum samples with a trace amount of hemolysis were rejected.
Biochemical analysisSerum endocan levels were measured with a commercially available kit for Endocan (human endothelial cell-specific molecule 1) (CUSABIO Lot No: 504228233) with enzyme-linked immunosorbent assays method (ELISA) (Awareness Chromate model ELISA machine, washer-reader, Awereness Stafax 2600 model, Awareness Technology Inc., Palm City, FL, USA). Results were stated as ng/mL for endocan.
Sepsis was diagnosed based on the criteria previously instituted by Gitto et al.14 A commercially available CRP kit (IMAGE device, the Beckman Coulter, sensitive value=0.8mg/dL) were used to measure serum C-reactive protein (CRP) levels by nephelometric method. And also, a commercially available Interleukin 6 (IL-6) kit (Siemens Healthcare Products Ltd, sensitivity value=2pg/mL) were chosen to evaluate serum IL-6 levels through solid phase enzyme labeled chemiluminescent immunometric assay method (Immulite 2000 device).
Statistical analysisStatistical analysis was applied by using the Statistical Package for Social Sciences (SPSS) (version 15 for Windows, SPSS Inc., St. Louis, MO). P value of .05 was considered significant. Non-parametric continuous variables for independent-samples were analyzed by using Student t-test and/or Mann–Whitney U-test, and categorical variables were analyzed by application of chi-square or Fisher's exact tests. Findings were stated as median (minimum–maximum) and/or mean ± standard deviation (SD) for continuous variables. In addition, categorical variables and distribution of frequency were presented as percentage. Receiver operating characteristic (ROC) analysis and the area under the curve (AUC) were applied to identify the diagnostic value of serum endocan levels in patients diagnosed with moderate and severe BPD.
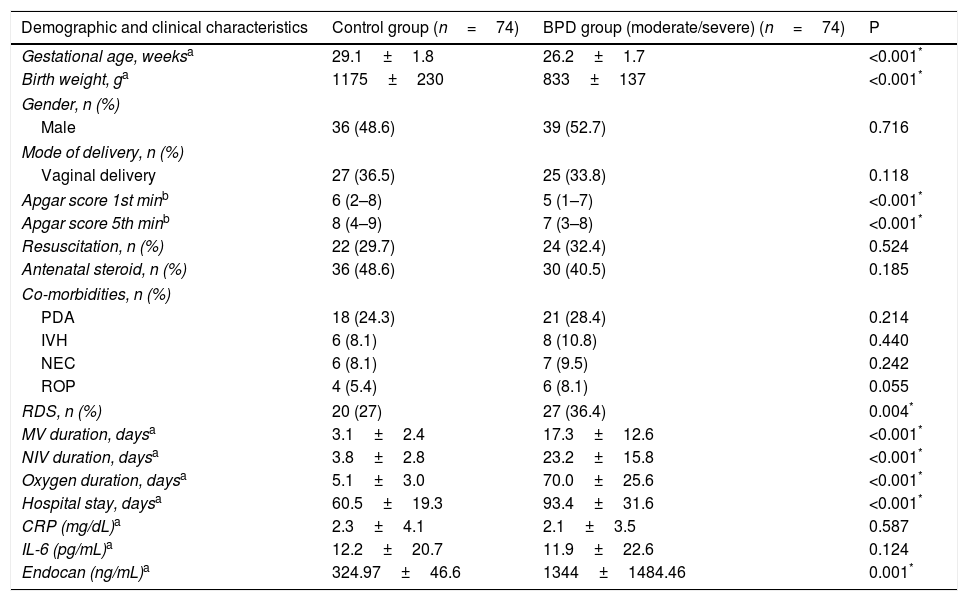
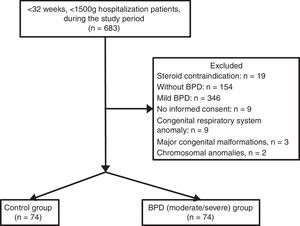
ResultsDuring the study period, 683 infants with VLBW and GA <32 weeks were evaluated. Infants whose parents did not sing the informed consent form to give permission (n=9), and infants with congenital respiratory system anomaly (congenital diaphragmatic hernia and congenital cystic adenomatoid malformation, n=2), mild BPD (n=346), and without BPD (n=178) were excluded. A final number of 148 infants were included. The groups were assigned as the control (n=74) and BPD (moderate/severe) (n=74). Flowchart of the eligible infants were shown in Fig. 1.
Mean GA and BW were 28.2±1.7 weeks and 994±202g, respectively. Birth weight, GA and Apgar scores (at the 1st and 5th min) were lower in the “BPD” group (P = .05). The duration of non-invasive ventilation support, mechanical ventilation therapy and oxygen support, RDS rates were higher in the “BPD” group (P = .05). The length of hospital stay was longer in the “BPD” group (P = .05). No significant differences were found between the BPD and control groups in terms of gender, modes of delivery, need for resuscitation, antenatal steroid treatment, and co-morbidities of infants (PDA, IVH, NEC, and ROP) (P = .05). No significant differences were determined between the control and study groups in terms of serum CRP and IL-6 levels (P = .05). However, A significant difference were shown between BPD and control groups in terms of endocan serum levels (P = .001) (Table 1).
Demographic And Clinical Characteristics Of The Groups.
| Demographic and clinical characteristics | Control group (n=74) | BPD group (moderate/severe) (n=74) | P |
|---|---|---|---|
| Gestational age, weeksa | 29.1±1.8 | 26.2±1.7 | <0.001* |
| Birth weight, ga | 1175±230 | 833±137 | <0.001* |
| Gender, n (%) | |||
| Male | 36 (48.6) | 39 (52.7) | 0.716 |
| Mode of delivery, n (%) | |||
| Vaginal delivery | 27 (36.5) | 25 (33.8) | 0.118 |
| Apgar score 1st minb | 6 (2–8) | 5 (1–7) | <0.001* |
| Apgar score 5th minb | 8 (4–9) | 7 (3–8) | <0.001* |
| Resuscitation, n (%) | 22 (29.7) | 24 (32.4) | 0.524 |
| Antenatal steroid, n (%) | 36 (48.6) | 30 (40.5) | 0.185 |
| Co-morbidities, n (%) | |||
| PDA | 18 (24.3) | 21 (28.4) | 0.214 |
| IVH | 6 (8.1) | 8 (10.8) | 0.440 |
| NEC | 6 (8.1) | 7 (9.5) | 0.242 |
| ROP | 4 (5.4) | 6 (8.1) | 0.055 |
| RDS, n (%) | 20 (27) | 27 (36.4) | 0.004* |
| MV duration, daysa | 3.1±2.4 | 17.3±12.6 | <0.001* |
| NIV duration, daysa | 3.8±2.8 | 23.2±15.8 | <0.001* |
| Oxygen duration, daysa | 5.1±3.0 | 70.0±25.6 | <0.001* |
| Hospital stay, daysa | 60.5±19.3 | 93.4±31.6 | <0.001* |
| CRP (mg/dL)a | 2.3±4.1 | 2.1±3.5 | 0.587 |
| IL-6 (pg/mL)a | 12.2±20.7 | 11.9±22.6 | 0.124 |
| Endocan (ng/mL)a | 324.97±46.6 | 1344±1484.46 | 0.001* |
BPD, bronchopulmonary dysplasia; CRP, C-reactive protein; IL-6, interleukin-6; IVH, intraventricular hemorrhage; MV, mechanical ventilation; NEC, necrotizing enterocolitis; NIV, non invasive ventilation; PDA, patent ductus arteriosus; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity.
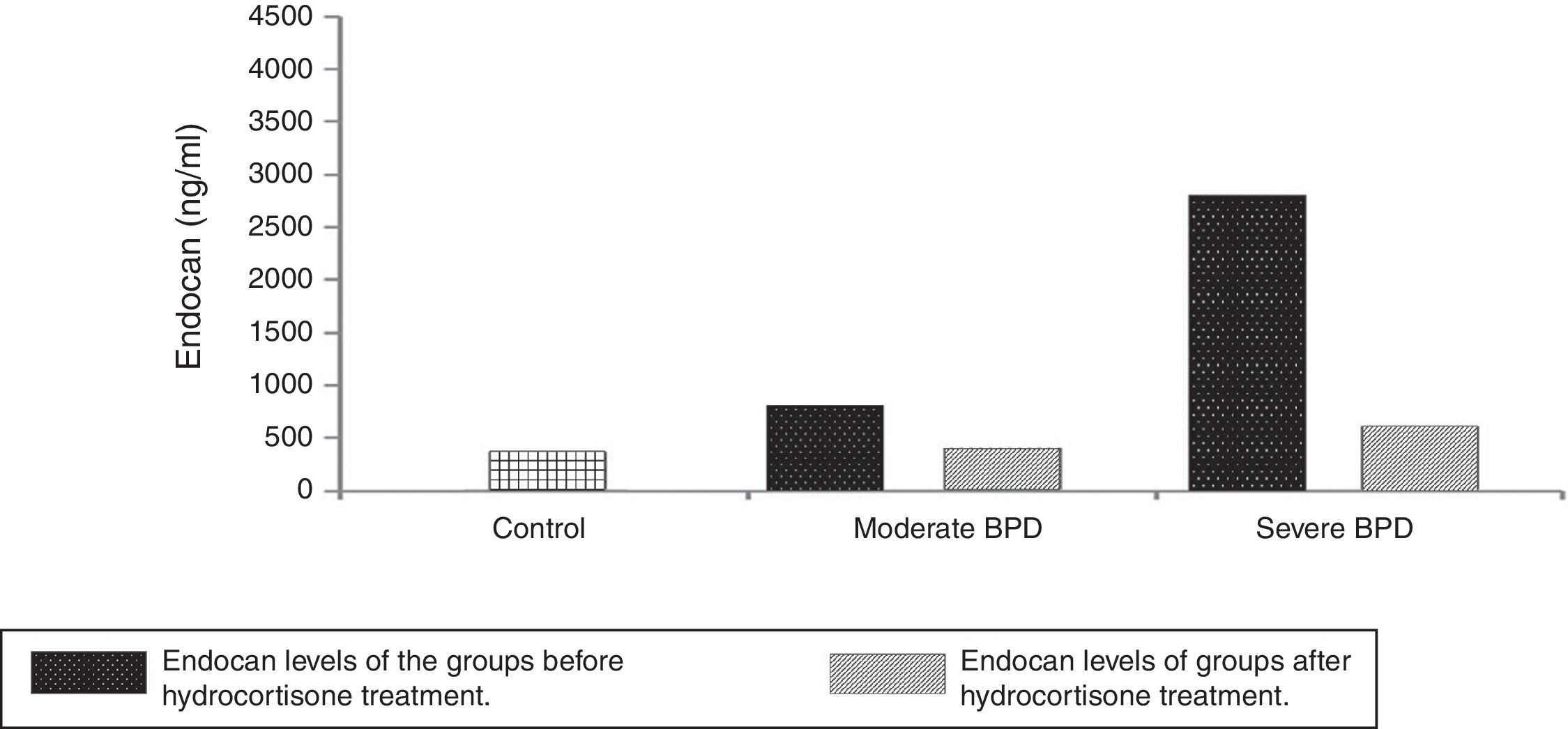
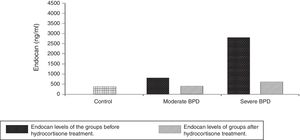
Infants in severe BPD group (n=28, 37.8%) had lower GA (25.2±1.1 weeks vs. 27.3±1.4 weeks) and lower BW (732±112g vs. 948±158g) compared to the infants in the moderate BPD group (n=46, 62.2%) (P = .000, P = .002, respectively). No significant differences were identified between control (287.34±132.54ng/mL) and BPD (293.25±116.47ng/mL) groups in terms of postnatal cord blood endocan levels (P = .564). Endocan levels were 324.97±46.6ng/mL in the control group. Endocan levels were determined to be higher in the severe BPD group (2947.66±1219.87ng/mL) than moderate BPD group (833.13±414.57ng/mL) (P = .000). Moreover, serum levels of endocan were remarkably higher in BPD groups than control group (P = .001). The mean serum endocan levels before decision of HC therapy at the time of diagnosis of moderate or severe BPD were significantly increased than these levels in the severe (496.4±283.75ng/mL) and moderate (346.51±168.34ng/mL) BPD after HC therapy (P = .004, P = .021, respectively). It was obvious that endocan values decreased significantly after medication of HC in infants with moderate or severe BPD. However, these levels for both severe and moderate BPD were still higher than control group (P = .05). It was determined that HC treatment decreased Endocan levels (Fig. 2).
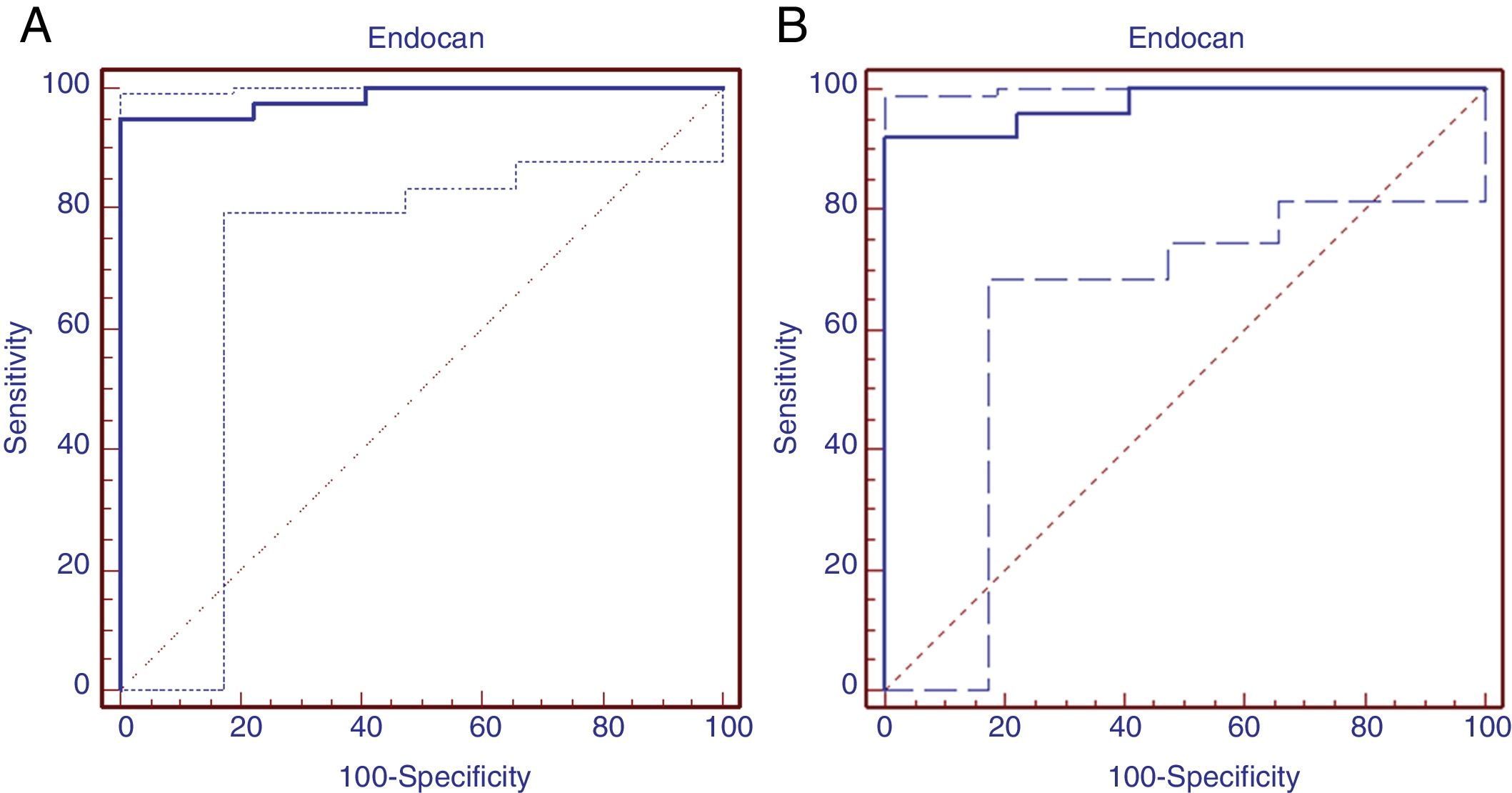
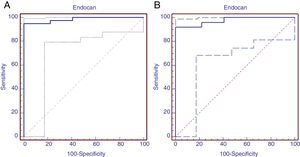
Receiver operating characteristic analysis was performed to determine the predictive diagnostic value of serum endocan levels in patients with moderate BPD. Area under the ROC curve was 0.984 (95% confidence interval=0.918–0.998, P = .0001). The cut-off value of Endocan was calculated as 395.93 with Youden index, sensitivity 95% (95% confidence interval=83–99.2), specificity 100% (95% confidence interval=87.1–100.0), positive predictive value 100.0% and negative predictive value 70.3%. (ROC curve was shown in Fig. 3A). ROC analysis was implemented to identify the diagnostic value of serum endocan levels in patients diagnosed with severe BPD. Area under the ROC curve was found to be 0.975 (95% confidence interval=0.888–0.997, significance level P = .001). The Endocan cut-off value was calculated to be 553.3 with the Youden index, sensitivity 92% (95% confidence interval=73.9–99.8), specificity 100% (95% confidence interval=86.7–100.0), positive predictive value 100.0% and negative predictive value 100% (Fig. 3B).
DiscussionTo our knowledge, the present research was the first that aimed to figure out the relevance of endocan for the diagnosis, follow-up and monitoring the therapy of BPD in preterm infants. Current results demonstrated that serum levels of endocan were increased in infants with BPD than the levels in the control group. Additionally, it was highlighted that serum levels of endocan were higher in infants with BPD before HC therapy and reduced throughout the consecutive days following appropriate therapy. Our results provided the idea that endocan could be recognized as a useful biomarker for the diagnosis and predicting the severity of BPD in addition to monitoring of treatment response.
Bronchopulmonary dysplasia occurs because of a complex process in which some prenatal and/or postnatal factors affect the development of the lower respiratory tract, resulting in severe, life-long adverse outcomes.15 In general, many studies state that BPD does not only reduce pulmonary function during infancy, but also increases the likelihood of developing asthma.16 The levels of different biomarkers have been measured in the blood as well as in tracheal aspirates in various studies, however, both blood and tracheal levels of these markers may not exactly reflect lung concentrations as well as distal lung parenchymal tissue.2 It is thought that BPD is caused by the effects of inflammatory processes on an immature and fragile lung. Therefore, researchers have studied on alterations in growth factors and inflammatory mediators related to development of alveolar, airway, or vascular structure that are effective in lung injury.2 However, many biomarkers are not unique to the disease alone, and appear to be not always effective in predicting the disease.17
In preterm infants, normal lung development deteriorates and interrupts with preterm delivery. Therefore, interrupted alveolar growth and maturation result in impaired or “dysmorphic” vascular growth. A decrease in the number of small arteries and abnormal vascular distribution in the distal lung has been demonstrated in various animal models and lung tissues in patients died because of BPD.18 As a result, abnormal and deteriorated angiogenesis appears to be the key to pathogenesis of the disease in patients with BPD. Abnormal vascular structures increase and continue in the formation of BPD as well as abnormal alveolar structures.19 It is a reality that in babies with BPD, both systemic and lung hypoxia occurs due to lung pathology and hypoxic episodes Furthermore, the depth of this hypoxia is also correlated with the severity of the disease.20,21 This means that vascular endothelial damage continues and rises according to severity of the disease.
Endocan is involved in the pathogenesis of many diseases that are associated with up-regulation of adhesion molecules, defined by microvascular inflammation and various degree of endothelial injury as well as lung diseases. Current data show that endocan might be effective in the pathogenesis of a wide spectrum of pulmonary disease ranging from atopic asthma, chronic obstructive pulmonary disease to lung cancer. Considering the selective expression of endocan in pulmonary endothelial cells activated in different lung diseases, suggest that increased endocan levels can reflect the severity of the pathophysiological conditions of the disease, together with the underlying endothelial dysfunction.6 In our study, endocan levels were found to be increased in patients with severe BPD. Thus, endocan originating from endothelium may also play a role in BPD accompanied by endothelium-dependent pathologic condition, and may be an endothelial dysfunction indicator that can be used to determine the severity of BPD.6,22
One recent study has provided that higher endocan levels are existed in patients with moderate-severe obstructive sleep apnea (OSA) on continuous positive airway pressure therapy. These results have determined that a good association is found between the severity of the disease in patients with OSA and endocan levels, in addition to distinguishing patients with OSA from patients who are suspected of OSA. These data supports the potential role of endocan as a biomarker in the identification and monitoring of affected individuals with OSA which has an endothelium-dependent pathology.22 Therefore, endocan has been proposed a valuable indicator for disease severity in patients who develop acute respiratory distress syndrome (ARDS) and postoperative pneumonia after cardiac surgery.23,24 One study conducted by Tang et al.25 has suggested that remarkably elevated plasma endocan levels are consisted with increased incidence of multiple organ dysfunctions in patients with pneumonia leading ARDS compared in patients without pneumonia. In few studies conducted in newborns have reported that endocan can be used as a marker in the diagnosis, follow-up and for the monitoring of response of the treatment of hemodynamic PDA and neonatal sepsis as well as estimating the severity of the diseases.23,24,26,27 In our study, the increase of endocan levels in infants with severe BPD and the higher level of endocan in BPD group than those in the control group suggested that endocan might be used both in the diagnosis and in determining severity of the disease. In addition, our results determined a remarkable decrease in endocan levels after hydrocortisone treatment. Therefore, it could be proposed that endocan may be suggested as a biomarker for the diagnosis and prediction of the severity of BPD, for making decision of appropriate therapy, and for monitoring response to therapy with together other clinical and laboratory findings. In the literature, there exist few markers associated with the severity of BPD in a small number of studies.28,29 In our study, infants with BPD had higher endocan levels than control. Elevation in serum endocan levels was significantly associated with severe or moderate BPD. Our results provide that endocan may be used as a reliable marker to diagnosis and follow up of BPD.
Pulmonary inflammation is defined as an important factor in the pathogenesis of BPD. As glucocorticoids have strong anti-inflammatory effects, hydrocortisone is used in the treatment of BPD. In our study, BPD severity decreased with anti-inflammatory effect of hydrocortisone. In addition, increased endocan levels that might strongly relate to vascular endothelial damage and inflammatory response were significantly reduced with HC treatment.30 Therefore, endocan can be suggested for monitoring of treatment response. The primary purpose of our study was to evaluate the potential candidates (moderate and severe BPD) for HC treatment. Therefore, serum endocan levels were not evaluated for mild BPD. In the present study, endocan levels were found to be similar at birth, regardless of gestational week and birth weight. However, due to the low GA and BW in the BPD group, the frequency of respiratory support, RDS and development of BPD were higher. Accordingly, postnatal factors were also important contributors for the development of BPD as well as prematurity.4,5,17 In general, it is difficult to obtain the biomarkers used to diagnose and to identify the severity of diseases. Based on our results, we would suggest that endocan can be considered as a potential marker for the severity of the disease in addition to be an effective indicator for monitoring the response to treatment.
Some markers from tracheal aspirate [(IL-1β, -6, -8, TNF-α, monocyte chemoattractant protein (MCP), nuclear factor kappa B (NFkB), neutrophil gelatinase-associated lipocalin (NGAL), MMP-9, transforming growth factor (TGF)-β1, L-selectin, Ang-2, endothelin-1, fibroblast growth factor-2, pepsin] with increased biomarkers in serum [interleukin (IL)-1β, -6, -8, -10, interferon (IFN)-γ, granulocyte colony stimulating factor (GCSF), endostatin, vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF) BB, placental growth factor (PGF), E selectin, and B-type natriuretic peptide (BNP)] were found to be associated with the development and severity of BPD. However, as far as we know, the markers used were markers obtained immediately after birth or on the follow-up.2 A recent study investigating the role of IL-33 in the lungs of neonatal mice with hyperoxia-induced BPD has declared that IL-33 is associated with BPD. Additionally, anti-IL-33 treatment showed a decrease in both IL-33 levels and the severity of BPD.31 However, there were no studies evaluating biomarker levels obtained at birth, before/after BPD treatment, and associated with BPD severity. In this respect, our study was the first to illuminate that issue.
There were some limitations in our study. It is clear that there are many pathophysiological mechanisms contributing to the development of BPD. Therefore, the similarity of endocan levels in both groups in cord blood showed that endocan could not be used in predicting which patient would have BPD. However, postnatal inflammatory process contributes significantly to the development of BPD. The results of our study showed that endocan which contributes to the inflammatory process might be effective in the development of BPD together with other inflammatory markers.
In conclusion, endocan levels were found to be higher and endocan levels were related to severity of BPD in patients identified BPD compared to control group. After successful HC treatment, the serum levels of endocan decreased appropriately close to the levels of the control group. As many biomarkers are contributors in the development of BPD. Endocan may be a new marker for BPD. Our findings suggested that the serum levels of endocan might be a beneficial tool for predicting the severity of BPD and monitoring of successful therapy. Additionally, endocan levels in cord blood are similar in both groups. Therefore, cord blood endocan levels are not suggested as a marker for predicting BPD at birth. However, further studies are warranted on this subject.
FundingThis work was supported by Zekai Tahir Burak hospital education planning and coordination committee (grant numbers: 62/2016).
Conflict of interestsThe authors declare that they have no conflicts of interest
The authors would like to thank all the nursing staff of neonatal intensive care unit in Health Science University Zekai Tahir Burak Maternity Teaching Hospital.