Community-acquired pneumonia (CAP) is a common serious infection. This study aimed to evaluate the prognostic utility of neutrophil count percentage (NCP) and neutrophil–lymphocyte ratio (NLR) in patients with CAP.
MethodsRetrospective study of hospitalized patients with CAP. Patients had a blood test at admission and 3–5 days after hospitalization (early-stage test). The main outcome variables were 30-day and 90-day mortality.
ResultsTwo hundred and nine patients were included. Patients who survived had significant reductions in both NCP and NLR between admission and the day 3–5 blood tests (from 85.8% to 65.4% for NCP and from 10.1 to 3.2 for NLR). Twenty-five patients died in the first 90 days. Patients who died had lower, non-significant reductions in NCP (from 84.8% to 74%) and NLR (from 9.9 to 6.9) and significantly higher early-stage NCP and NLR than those who survived. NCP values higher than 85% and NLR values higher than 10 in the early-stage blood test were associated with a higher risk of mortality, even after multivariate adjustment (HR for NCP: 12; HR for NLR: 6.5).
ConclusionNCP and NLR are simple, low-cost parameters with prognostic utility, especially when measured 3–5 days after CAP diagnosis. High NLR and/or NCP levels are associated with a greater risk of mortality at 90 days.
La neumonía adquirida en la comunidad (NAC) es una infección frecuente y grave. El objetivo de este trabajo es estudiar la utilidad pronóstica del porcentaje de neutrófilos (NCP) y del cociente neutrófilos/linfocitos (NLR) en pacientes con NAC.
MétodosEstudio retrospectivo de pacientes hospitalizados por NAC con analítica al ingreso y una segunda extracción de control a los 3-5 días. Se consideraron variables desenlace la mortalidad a 30 y 90 días.
ResultadosSe incluyó a 209 pacientes. Los pacientes que sobrevivieron redujeron significativamente el NCP y el NLR entre la analítica al diagnóstico y la de control (desde el 85,8 hasta el 65,4% para NCP y de 10,1 a 3,2 para NLR). Fallecieron 25 pacientes en los primeros 90 días. En ellos hubo un menor descenso no significativo para el NCP (del 84,8 al 74,0%) y para NLR (de 9,9 a 6,9). Los valores de NCP y NLR en la analítica de control fueron significativamente mayores en los pacientes fallecidos que en los supervivientes. Aquellos pacientes que presentaron en la analítica de control un NCP superior al 85% o un NLR superior a 10, presentaron un riesgo de mortalidad superior tras ajuste multivariable (HR para NCP 12 y para NLR 6,5).
ConclusiónNCP y NLR son parámetros sencillos y de bajo coste, con utilidad pronóstica especialmente al medirse a los 3-5 días del diagnóstico de NAC. Niveles altos de NLR o NCP se asocian con mayor riesgo de mortalidad a los 90 días.
Community-acquired pneumonia (CAP) is a common infection that is potentially deadly.1,2 One of the objectives in the care of CAP patients at the time of diagnosis is to establish an estimated prognosis, in order to determine the need for hospitalization or for planning the most suitable follow-up. Numerous scales for assessing CAP severity and risk are available for this purpose, such as the CURB65 criteria (confusion, urea, respiratory rate, blood pressure, age>65 years),3 the Pneumonia Severity Index (PSI),4 and the Severe Community Acquired Pneumonia (SCAP) score.5 These scales have been widely validated in large population cohorts and are currently the most useful tools available for assessing the prognosis of CAP patients at the time of diagnosis.6–8
Efforts are currently underway to improve the prognostic value of these clinical scales. Given that pneumonia is a localized infectious process that causes a systemic inflammatory response, it is postulated that the study of this inflammatory process would assist in evaluating the severity of CAP and predicting its progress. In this respect, several biomarkers determined at the time of CAP diagnosis have been studied, including procalcitonin (PCT), proadrenomedullin (proADM) and copeptin9–14; these molecules measured at diagnosis have shown a greater prognostic power than C-reactive protein (CRP) or total leukocyte count, but have not proven to be superior to traditional scales. Several authors have reassessed the use of simpler, more accessible markers at diagnosis, such as the neutrophil/lymphocytes ratio (NLR)15,16 or the neutrophil count percentage (NCP),17 with the advantage that both are easily identifiable, inexpensive parameters. Along these lines, Curbelo et al. compared NCP with PCT, proADM, and copeptin, and found it was not significantly inferior in terms of its prognostic capacity for mortality in the short and medium term.
Continuing in the search for efficient biomarkers, other authors have proposed simple, economic parameters and evaluated their usefulness not only for diagnosis, but also for the follow-up of patients with CAP. Zhydkov et al. found that total leukocyte counts and CRP in patients hospitalized for CAP provided prognostic information if they were evaluated in clinical laboratory tests during patient follow-up.18 Other studies also show that NLR and NCP in early-stage blood tests (at 3–5 days) are equally or more useful than determinations made on admission,17 and suggest that these 2 parameters may be very useful prognostic markers of the progress of patients hospitalized for CAP.
The aim of this study was to evaluate the usefulness of NCP and NLR measured during the course of CAP, and their role as predictors of mortality at 30 and 90 days.
Materials and MethodsThis was a retrospective study of CAP patients admitted to the respiratory medicine or internal medicine department of the Hospital Universitario de La Princesa in Madrid between 2010 and 2012. The study protocol was approved by the Clinical Research Ethics Committee of the same center. Provisions for data collection reflected in the applicable legislation, Act 15/1999 on Personal Data Protection, were followed.
The inclusion criteria were: age>18 years at the time of diagnosis and hospitalization for CAP in the departments of Respiratory Medicine or Internal Medicine. We determined that both the admission and discharge reports listed the primary diagnosis as CAP, and the presence of lower respiratory tract symptoms (cough, expectoration, dyspnea, tachypnea or pleuritic pain) and the appearance of a new infiltrate on X-ray without any other justifiable cause.19 Only patients with clinical laboratory tests performed at the time of CAP diagnosis and at least once more before hospital discharge were included. This second test was the one performed 3–5 days after the date of admission. Total leukocytes and differential counts were determined from peripheral blood in EDTA, and by fluorescence flow cytometry and hydrodynamic focusing (forward and side scatter with a Sysmex XE-2100™ automated hematology analyzer (Sysmex, Kobe, Japan).
We excluded patients who did not receive antibiotics in the context of a decision to limit treatment. Exclusion criteria included the presence of active hematologic or oncologic disease and severe immunodeficiency (transplant recipients or patients with human immunodeficiency virus infection with <500 CD4+).
All patients were classified using the PSI and CURB65 assessment scales, and their sociodemographic characteristics, comorbidities, and treatment were systematically recorded. We calculated the modified Charlson comorbidity index proposed by Bordón et al. for patients with CAP.20 In addition, clinical, radiological, and laboratory variables associated with the CAP episode were recorded. The definitions of some of these diseases are given in the Annex. Patients were treated according to the routine clinical practice, following the therapeutic recommendations of the main European clinical guidelines. The main outcome variable was all-cause death 30 and 90 days after CAP diagnosis.
Statistical AnalysisDifferences between qualitative variables were analyzed using the Chi-squared test or, in the case of expected frequencies lower than 5, Fisher's exact test. Differences in the quantitative variables were analyzed using the Student's t-test or, in the case of non-normally distributed variables, the non-parametric Wilcoxon test. An inter-subject analysis was performed to assess changes in NCP and NLR between the determinations at diagnosis and follow-up.
We analyzed the predictive power of NCP and NLR on 90-day mortality by calculating areas under the ROC curve (AUC). We also analyzed the usefulness of cut-off points for NCP and NLR proposed in the literature; in the case of NLR, the usefulness of the cutoff point of 10 proposed by de Jager et al.15 was analyzed. For NCP, the cutoff point of 85 proposed in previous articles by our research group17 was analyzed. For this evaluation, we calculated the hazard ratio (HR) or incidence ratio of mortality as a function of NCP and NLR; subsequently, a multivariate adjustment was made using the Cox regression model, including the variables that showed differences with P<0.10, applying clinical criteria to obtain a parsimonous model.
For the calculation of the sample size, we used data from the gold-standard prospective study.17 In that study, patients with NCP>85% in the early-stage test had a 90-day mortality rate of 55% compared to the group with NCP<85%, who had a mortality rate of 7.5%. The ratio between the two groups was 1:12, so for an alpha error of 5% and a power of 95%, a sample size of 173 subjects was required. Similar results were obtained for the NLR cutoff point of 10 in the early-stage test. Based on these data, a target sample size of 200 subjects was established.
Probability values were considered statistically significant at P<0.05. The statistical analysis was carried out with Stata software (version 13.1; Stata Corporation, College Station, Texas, USA).
ResultsA total of 275 patients were analyzed in a consecutive and undirected manner. Twenty-eight episodes were excluded because of readmissions and 38 because of unavailable blood tests at 3–5 days. The final sample was composed of 209 patients, in order to reach the estimated sample size (200 patients).
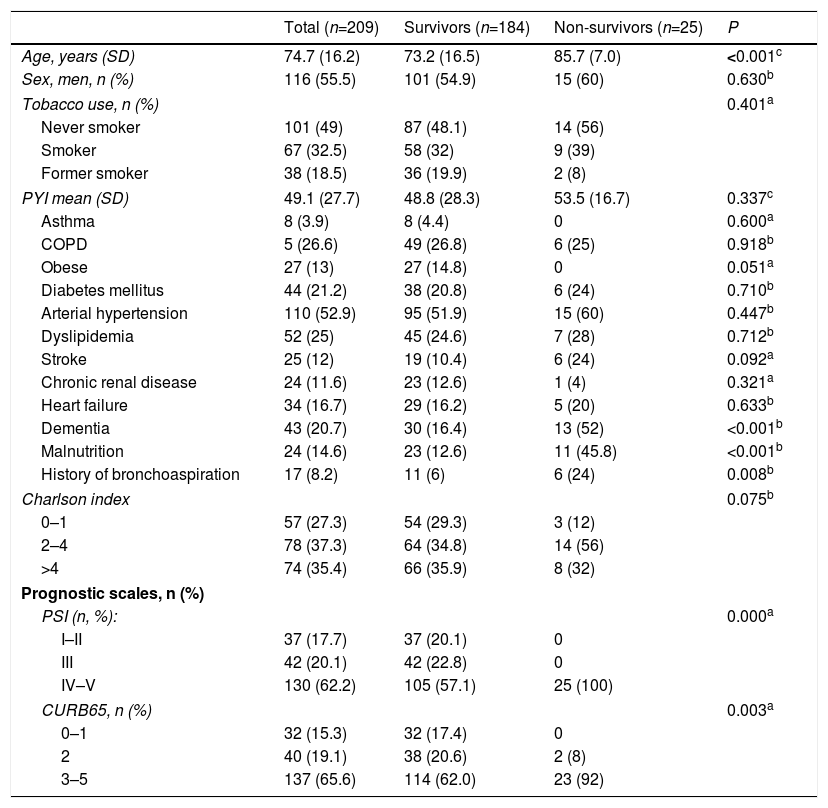
Nine patients (4.3%) died during hospitalization (95% confidence interval [CI]: 2.3–8), whereas 5.7% had died by day 30 (95% CI: 3.3–9.8). Ninety days after diagnosis, 25 patients had died, with a cumulative mortality of 12% (95%: 8.2–17.1) Baseline characteristics of the study population and differences between those who had died and those who survived at 90 days can be found in Table 1. Patients who died had a higher average age (85.7 years vs 73.2; P<0.001). This subgroup of patients showed a prevalence of cognitive impairment, malnutrition, and a significantly greater history of bronchoaspiration. Comorbidity measured by the Charlson index was slightly higher in patients who died; in that group, 88% had an index of 2 or higher, compared to 70.7% in the group of survivors; however, this difference did not reach statistical significance (P=0.075). Moreover, patients who progressed adversely had a significantly higher level of CAP severity evaluated by PSI or CURB65.
Baseline Characteristics of the Study Population by 90-Day Mortality.
| Total (n=209) | Survivors (n=184) | Non-survivors (n=25) | P | |
|---|---|---|---|---|
| Age, years (SD) | 74.7 (16.2) | 73.2 (16.5) | 85.7 (7.0) | <0.001c |
| Sex, men, n (%) | 116 (55.5) | 101 (54.9) | 15 (60) | 0.630b |
| Tobacco use, n (%) | 0.401a | |||
| Never smoker | 101 (49) | 87 (48.1) | 14 (56) | |
| Smoker | 67 (32.5) | 58 (32) | 9 (39) | |
| Former smoker | 38 (18.5) | 36 (19.9) | 2 (8) | |
| PYI mean (SD) | 49.1 (27.7) | 48.8 (28.3) | 53.5 (16.7) | 0.337c |
| Asthma | 8 (3.9) | 8 (4.4) | 0 | 0.600a |
| COPD | 5 (26.6) | 49 (26.8) | 6 (25) | 0.918b |
| Obese | 27 (13) | 27 (14.8) | 0 | 0.051a |
| Diabetes mellitus | 44 (21.2) | 38 (20.8) | 6 (24) | 0.710b |
| Arterial hypertension | 110 (52.9) | 95 (51.9) | 15 (60) | 0.447b |
| Dyslipidemia | 52 (25) | 45 (24.6) | 7 (28) | 0.712b |
| Stroke | 25 (12) | 19 (10.4) | 6 (24) | 0.092a |
| Chronic renal disease | 24 (11.6) | 23 (12.6) | 1 (4) | 0.321a |
| Heart failure | 34 (16.7) | 29 (16.2) | 5 (20) | 0.633b |
| Dementia | 43 (20.7) | 30 (16.4) | 13 (52) | <0.001b |
| Malnutrition | 24 (14.6) | 23 (12.6) | 11 (45.8) | <0.001b |
| History of bronchoaspiration | 17 (8.2) | 11 (6) | 6 (24) | 0.008b |
| Charlson index | 0.075b | |||
| 0–1 | 57 (27.3) | 54 (29.3) | 3 (12) | |
| 2–4 | 78 (37.3) | 64 (34.8) | 14 (56) | |
| >4 | 74 (35.4) | 66 (35.9) | 8 (32) | |
| Prognostic scales, n (%) | ||||
| PSI (n, %): | 0.000a | |||
| I–II | 37 (17.7) | 37 (20.1) | 0 | |
| III | 42 (20.1) | 42 (22.8) | 0 | |
| IV–V | 130 (62.2) | 105 (57.1) | 25 (100) | |
| CURB65, n (%) | 0.003a | |||
| 0–1 | 32 (15.3) | 32 (17.4) | 0 | |
| 2 | 40 (19.1) | 38 (20.6) | 2 (8) | |
| 3–5 | 137 (65.6) | 114 (62.0) | 23 (92) | |
COPD: chronic obstructive pulmonary disease; PSI: Pneumonia Severity Index; PYI: pack-year index in smokers and ex-smokers; SD: standard deviation.
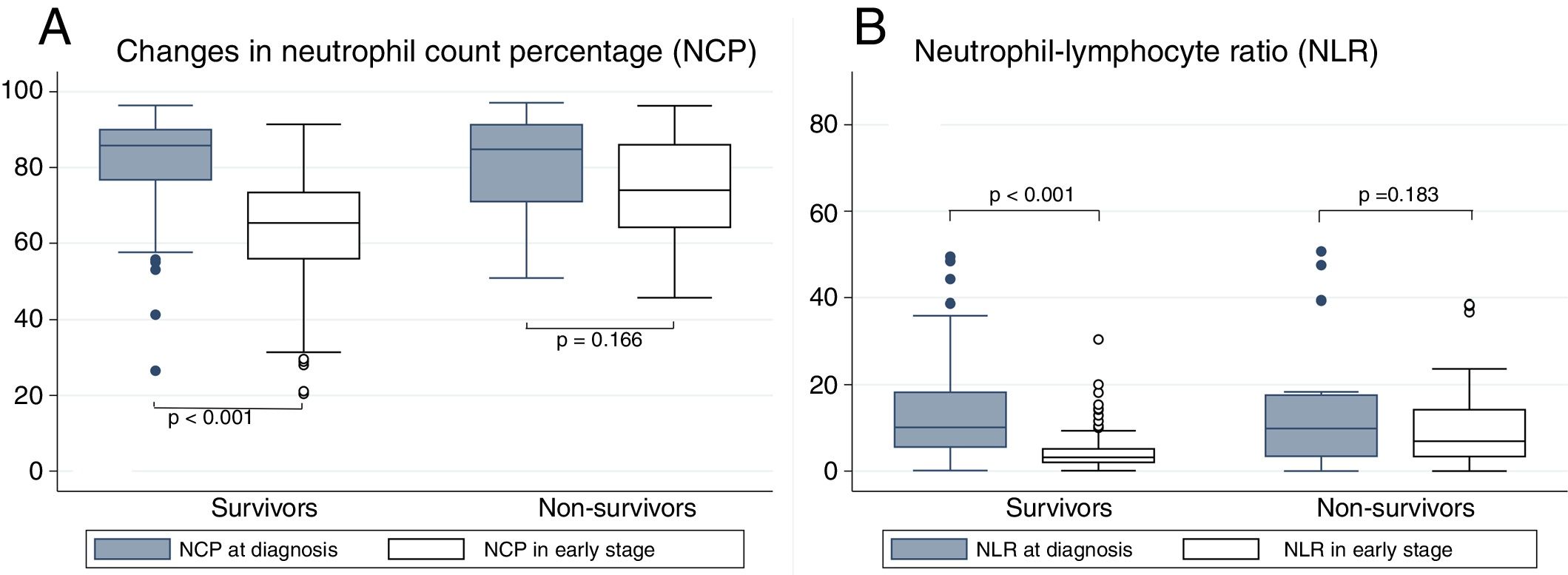
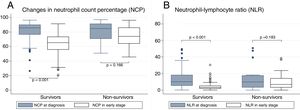
The analysis of the relationship between NCP and NLR with mortality is shown in Fig. 1. It can be seen that patients who died and those who progressed well showed similar NCP values in their tests on admission (84.8% vs 85.8%, P=0.794) and the same occurs with NLR (9.9 vs 10.1; P=0.652). In contrast, in the early early-stage test, significant differences were found between the non-survivors and the survivors for both NCP (74% vs 65.4%, P<0.001) and NLR (6.9 vs 3.2; P<0.001). When intra-subject progress is analyzed, patients who survived are seen to present a significant reduction in NCP between tests on admission and early-stage tests (−19.1, P<0.001); the same is true of NLR (−6.9, P<0.001). However, while the non-survivors experienced a decrease in their NCP (−4.4), this reduction was not significant (P<0.166). The same situation occurred with NLR (reduction of −1.6 with P=0.183).
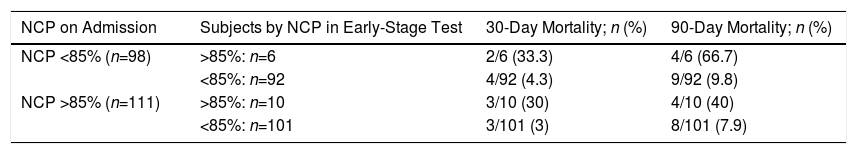
On the basis of the cutoff points proposed in previous studies, patients were stratified according to their NCP and NLR levels at admission or in early-stage tests: NCP>85% or NLR>10 (Table 2). The analysis of NCP shows that patients with values>85% at admission and at discharge had a mortality rate at 30 days of 30%, and 40% at 90 days. On the other hand, subjects with NCP<85% at admission but whose NCP in the early-stage test subsequently rose to >85% had a 30-day mortality rate of 33.3% and 66.7% at 90 days. At the other extreme, patients who had levels persistently lower than 85%, or those who began with high levels that then fell, had significantly lower 30-day (4.3 and 3%, respectively) and 90-day (9.8% and 7.9%) mortality rates. A similar situation occurs when the progress of NLR is analyzed.
Analysis of 30-Day and 90-Day Mortality by the Neutrophil Count Percentage (NCP) and Neutrophil/Lymphocyte Ratio (NLR).
| NCP on Admission | Subjects by NCP in Early-Stage Test | 30-Day Mortality; n (%) | 90-Day Mortality; n (%) |
|---|---|---|---|
| NCP <85% (n=98) | >85%: n=6 | 2/6 (33.3) | 4/6 (66.7) |
| <85%: n=92 | 4/92 (4.3) | 9/92 (9.8) | |
| NCP >85% (n=111) | >85%: n=10 | 3/10 (30) | 4/10 (40) |
| <85%: n=101 | 3/101 (3) | 8/101 (7.9) |
| NCP at Admission | Subjects by NLR in Early-Stage Test | 30-Day Mortality; n (%) | 90-Day Mortality; n (%) |
|---|---|---|---|
| NLR<10 (n=104) | >10: n=8 | 2/8 (25) | 3/8 (37.5) |
| <10: n=96 | 4/96 (4.2) | 10/96 (10.4) | |
| NLR>10 (n=105) | >10: n=11 | 4/11 (36.4) | 5/11 (45.5) |
| <10: n=94 | 2/94 (2.1) | 9/94 (7.4) |
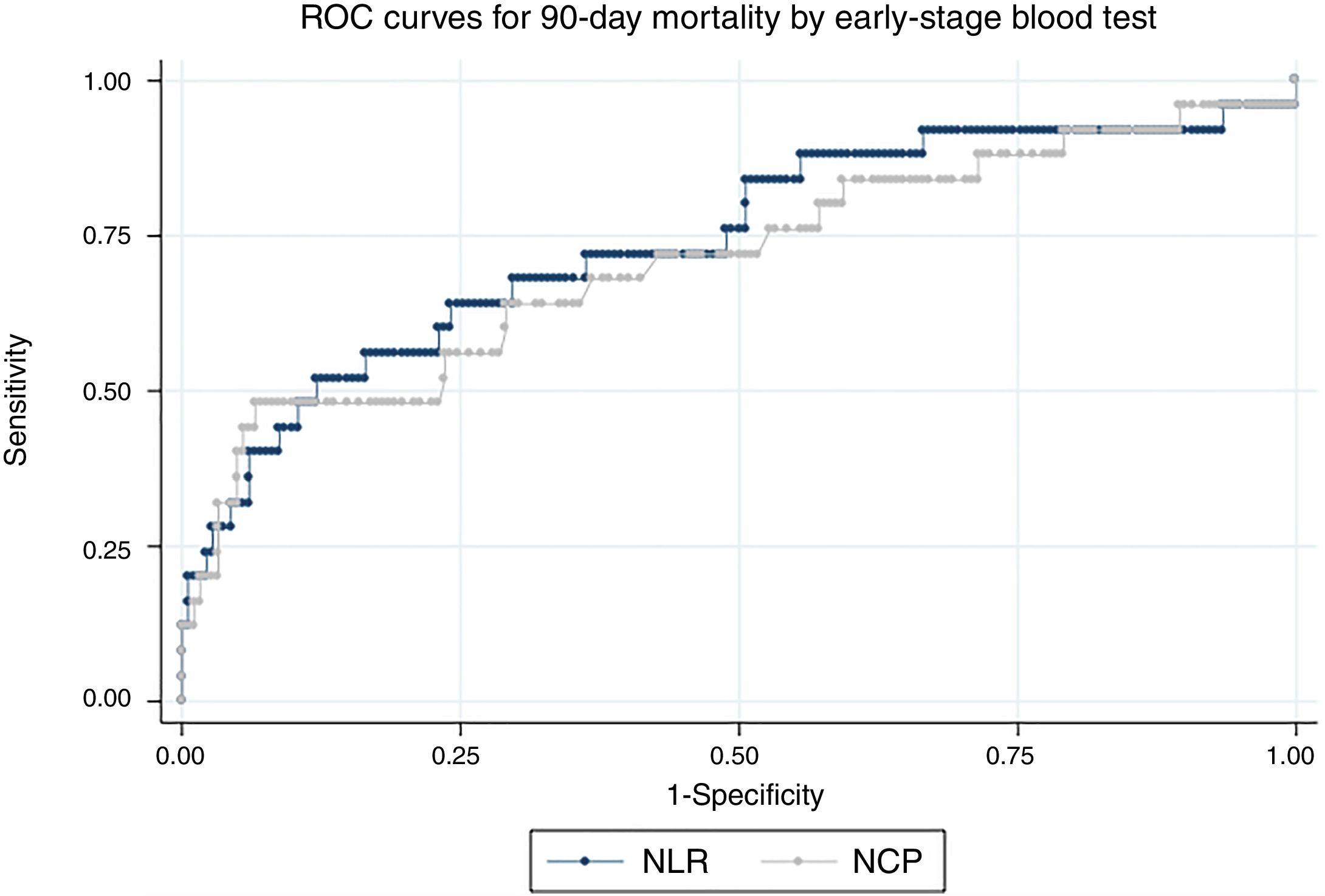
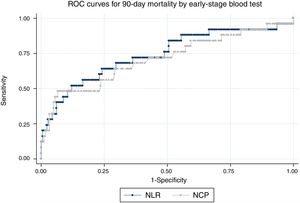
Fig. 2 shows the ROC curves for NCP and NLR in the early-stage blood tests for 90-day mortality. The AUC for NCP was 70.9 (95% CI: 58.3–83.6) and 74 for NLR (95% CI: 62.1–86) For 30-day mortality, the AUC of NCP in the early-stage test was 84 (95% CI: 72.1–96) and 88.3 for NLR (95% CI: 79.4–97.2)
With regard to the assessment tools, the AUC for the CURB65 score was 68.4 (95% CI: 59.4–77.5) for 90-day mortality and 69.8 (95% CI: 59.2–80.4) for 30-day mortality. The AUC of the PSI score for 90-day mortality was 76.7 (95% CI: 69.9–83.6) and 78.4 (95% CI: 69–87.8) for 30-day mortality. None of the scores was statistically superior to NLR or NCP values in the early-stage blood test.
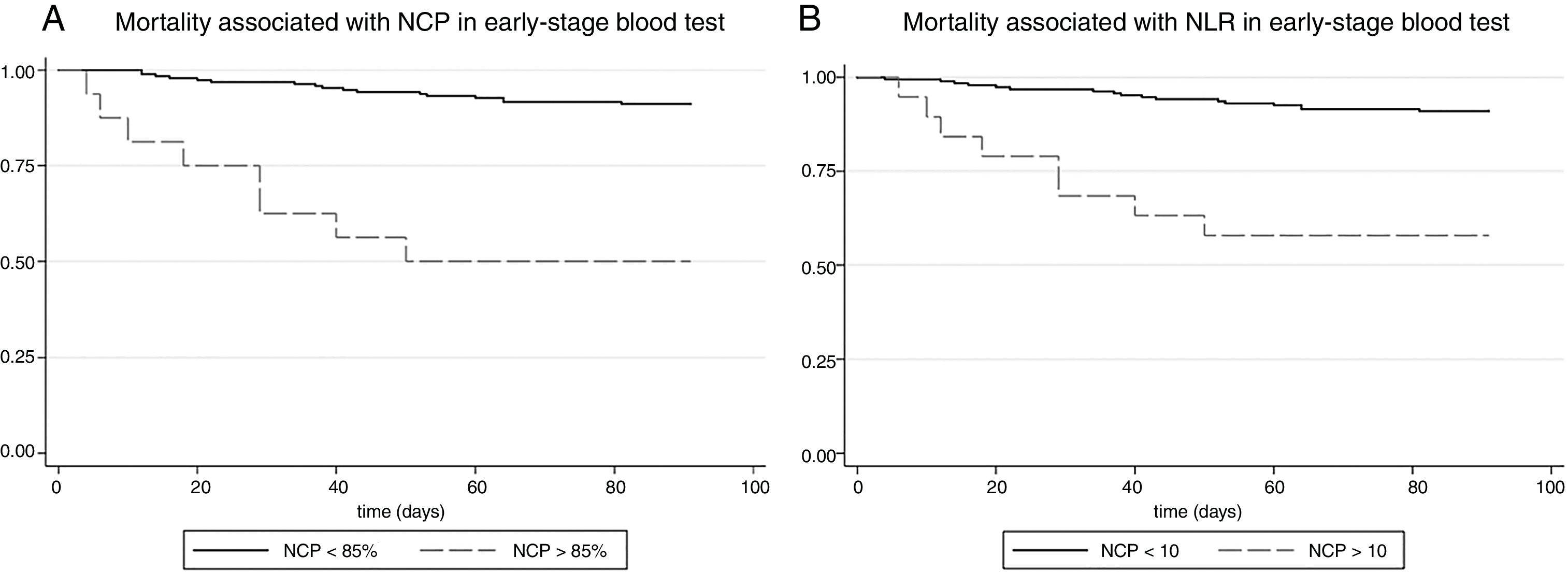
To assess the predictive power of these parameters in the early-stage blood test, the HR for 90-day mortality was calculated. Thus, NCP>85% in the early-stage test was accompanied by an HR for 90-day mortality of 8.16 (95% CI: 3.5–19), that is to say, an 8-fold mortality risk compared to patients with NCP<85%. Fig. 3 shows mortality as a function of NCP. After adjusting for age, sex, malnutrition, a history of stroke, cognitive impairment, a history of bronchoaspiration, and CAP severity evaluated by CURB65, the HR was 12 (95% CI: 4.3–33.3), that is to say, statistical significance was maintained. In the analysis of the difference in NCP between admission and early-stage blood tests, a reduction of 10 units was associated with a statistically significant adjusted HR of 0.67 (0.50–0.90). That is to say, subjects who had achieved a 10% reduction in NCP during follow-up had a risk of mortality one-third lower than those whose NCP did not fall.
The situation was similar in the case of the NLR (Fig. 3, panel B). A value of NLR>10 in the early-stage test was accompanied by a univariate HR for 90-day mortality of 6.1 (95% CI: 2.6–14.2), and after multivariate adjustment, the HR, at 6.5, was statistically significant (95% CI: 2.5–16.7). In the analysis of the difference of NLR between admission and early-stage blood tests, a reduction of NLR of 5 units was associated with a statistically significant adjusted HR of 0.83 (0.70–0.98).
The analysis of 30-day mortality showed similar results: after multivariate adjustment, the HR for the NCP cutoff point of 85% in the early-stage blood test was 18.3 (95% CI: 3.8–88.6) and 9.9 for the NLR cutoff of 10 (95% CI: 2.5–38.7). In this case, a reduction of 10 percentage points from the baseline NCP value in the early-stage test compared to the value at admission was accompanied by an HR of 0.5 (95% CI: 0.3–0.9) Similarly, a reduction in NLR of 5 units in the respective tests was associated with an HR of 0.8 (95% CI: 0.6–0.9)
DiscussionThis series of patients hospitalized for CAP demonstrates the prognostic value of NCP and NLR. High levels of NCP and NLP at admission are poorer markers of mortality than persistently high or rising values during hospitalization. The presence of NCP>85% or NLR>10 in the early-stage blood test is associated with a high risk of mortality regardless of age, sex, comorbidities, or CAP severity evaluated by the conventional scores.
Patients who do not survive do not show a significant reduction in NCP or NLR over the course of their disease, while those with initially low levels experience an increase in these markers. This finding suggests that an uncontrolled immune response, in which neutrophils predominate over other leukocytes (NCP) or over the lymphocyte population (NLR), could be a sign of persistent and ineffective immune response indicative of adverse clinical outcomes. Zhydkov et al. performed blood tests at admission, on days 3, 5, and 7, and at discharge.18 In their study, they demonstrated the prognostic value not only of PCT, but also of the total leukocyte count and CRP, less so in the initial tests, and markedly in the early-stage tests. Unfortunately, in this study NCP and NLR data were not provided, and AUC for the isolated markers were not calculated. In previous studies, our group performed 2 blood tests in a prospective cohort of patients with CAP requiring hospitalization and demonstrated the utility of parameters such as NLR and NCP at admission, but especially in early-stage tests, finding a prognostic power comparable to that of other molecules, such as proADM, PCT, and copeptin.17 This study strengthens the evidence provided by these previous papers, stressing the usefulness of blood tests as part of the early-stage monitoring of progress in patients with severe CAP requiring hospitalization.
There is abundant evidence to show that PCT is the most useful biomarker for monitoring the progress of respiratory infections. Normalization of PCT levels has been correlated with clinical improvement in patients with pneumonia.21 Furthermore, several clinical trials show that clinical management guided by levels of circulating PCT in patients with lower respiratory tract infections may lead to a reduction in the duration of antibiotic treatment,22,23 although this latter assertion is controversial.24,25 None of these studies has compared PCT with NCP or NLR. The few publications that directly compare PCT with NLR or NCP are observational studies, in which NCP and NLR show no inferiority when measured at admission or in early-stage tests.17 This study supports the results obtained by other groups on the usefulness of NCP and NLR as prognostic markers in the course of the infectious process. In addition, its findings raise the possibility that monitoring patients on the basis of these simple parameters may be sufficient, with the consequent cost savings.
Both the CURB65 and PSI indices help stratify the risk of CAP patients at the time of diagnosis, and no biomarker has consistently shown superiority over these scales. After diagnosis, the only indicator for patients with severe CAP available to the clinician is the course of the disease itself. In the subgroup of patients with severe CAP, the determination of NCP or NLR in early-stage blood tests would help identify patients at increased risk for adverse outcomes and indicate the need to intensify monitoring and plan close observation. It should be pointed out that while the NLR or NCP AUCs for 30-day and 90-day mortality in the early-stage blood test are similar to those of CURB65 or PSI, they are not comparable. The CAP assessment scales stratify the risk at the time of diagnosis, while NLR or NCP becomes useful in the post-acute phase, after the start of antibiotic treatment.
This study follows the lines of other authors who suggest the need to use new biomarkers in clinical practice. Simple, low-cost parameters, such as NCP or NLR, can provide clinically useful information. Moreover, as most studies compare biomarkers with CRP or total leukocyte counts, it seems reasonable that any new biomarker must be compared with these 2 parameters.
The main limitations of this study are its retrospective, single-centre design. Moreover, the cohort of CAP patients was restricted to those who were hospitalized and presented clinical laboratory results, so the full spectrum of severity of the disease is not represented. The inclusion of hospitalized subjects implies the exclusion of patients with mild CAP who would receive outpatient management. Furthermore, the criterion of requiring an early-stage blood test meant that some patients with a severe disease course and early death might have been excluded. Another potential limitation is the fact that the blood tests were carried 3–5 days after admission instead of on a specific day. Although this factor might generate a certain degree of imprecision, it provides the study with a degree of pragmatism and reflects clinical practice: protocolized blood tests performed on fixed days are unlikely to be translatable to day-to-day care.
ConclusionsNCP and NLR are accessible, inexpensive parameters that provide information on the prognosis of patients with severe CAP when analyzed in early follow-up, within 3–5 days following a diagnosis of pneumonia. High NLR or NCP values after several days of hospitalization for CAP are associated with an increased risk of 90-day mortality, regardless of comorbidities and the severity of the pneumonia.
FundingThis study was funded by the Instituto de Salud Carlos III (European Regional Development Fund) (PI 12/01142 and PI 15/01311) and by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR 89/2013 and SEPAR 98/2016).
Conflict of InterestsThe authors state that they have no conflict of interests.
Dementia. Dementia is a clinical syndrome characterized by global acquired memory and intellect impairment and personality disorder occurring in an alert, awake individual. For this study, a patient was considered to have dementia when his/her score on the Lobo's Mini Examen Cognoscitivo (MEC-30, Spanish equivalent of the Mini-Mental State Examination) was less than 24 or when his/her score on the Global Dementia Score (GDS) was greater than or equal to 4.
Malnutrition. Malnutrition is defined as the alteration of the body composition due to absolute or relative deprivation of nutrients that produces a decrease in nutritional parameters below the third percentile. For the purposes of classification, subjects with COntrolling NUTritional status (CONUT) scores greater than or equal to 5 (risk of moderate or severe malnutrition) were considered to be malnourished.
History of bronchoaspiration. Bronchoaspiration is the abnormal passage of fluid, exogenous substances or endogenous secretions to the lower airways. In this study, a patient was considered to have a history of bronchoaspiration in 2 situations: (1) patients with at least 1 episode of chemical pneumonitis or infectious pneumonia following an episode of bronchoaspiration witnessed by family members or caregivers; (2) patients with neurodegenerative diseases, cerebrovascular disease or fragility, with high risk of dysphagia, who had previously performed an ENT test for dysphagia with a positive result, despite not having presented witnessed bronchoaspirative episodes.
Please cite this article as: Curbelo J, Rajas O, Arnalich B, Galván-Román JM, Luquero-Bueno S, Ortega-Gómez M, et al. Estudio del porcentaje de neutrófilos y el cociente de neutrófilos-linfocitos como marcadores pronósticos en pacientes hospitalizados por neumonía adquirida en la comunidad. Arch Bronconeumol. 2019;55:472–477.