The etiology of lung cancer is multifactorial. Exposure to tobacco smoke and the role played by the carcinogenic compounds that it contains would explain the common association between lung cancer and chronic obstructive pulmonary disease (COPD), which is closely linked to tobacco use. In both diseases, sustained inflammation is caused by increased oxidative stress (for example, lipid peroxidation). This generates low molecular weight substances called volatile organic compounds (VOC) that are excreted during breathing. VOC metabolomics provides an indirect measure of oxidative stress.
ObjectiveThe aim of this study was to establish the relative influence of COPD on the VOC profile in patients with non-small cell lung cancer (NSCLC), by first studying the possible variation of VOC associated with lung cancer histology.
Patients and methodsExhaled air was tested in 107 NSCLC patients, who were divided into 2 groups: NSCLC with COPD and non-COPD with NSCLC. The exhaled air sample was obtained with the BIOVOC® sampler, and transferred to desorption tubes for later analysis by thermal desorption-gas chromatography-mass spectrometry. The VOC analysis showed lineal aldehydes and carboxylic acids.
Results and conclusionsNo statistically significant differences were found in VOC associated with histology. NSCLC and COPD patients present a 1.7-fold (1.1–2.7) greater probability of detection of propionic acid (95% CI: 1.22–6.2) than patients without COPD or NSCLC (p = 0.013).
La etiología del cáncer de pulmón es multifactorial. La exposición al humo del tabaco y el papel que desempeñan los compuestos carcinógenos presentes en él explicarían la frecuente asociación de cáncer de pulmón con enfermedad pulmonar obstructiva crónica (EPOC), enfermedad igualmente muy ligada al tabaquismo. En ambas entidades se desencadena una inflamación mantenida debida al incremento del estrés oxidativo (por ejemplo, peroxidación lipídica), que genera sustancias de bajo peso molecular llamadas compuestos orgánicos volátiles (VOC), que son excretadas durante la respiración. La metabolómica de los VOC ofrece una medida indirecta del grado de estrés oxidativo.
ObjetivoEl objetivo de este trabajo es determinar la influencia relativa de la EPOC en el perfil de los VOC en pacientes con cáncer de pulmón no microcítico (CPNM), estudiando previamente la posible variación de los VOC en función de la histología.
Pacientes y métodosSe analizó el aire exhalado de 107 pacientes de CPNM, clasificados en 2 grupos: CPNM con EPOC y CPNM sin EPOC. La muestra de aire exhalado se recogió mediante BioVOC® y se traspasó a tubos de desorción para su posterior análisis por cromatografía de gases y espectrometría de masas. Los VOC analizados fueron aldehídos lineales y ácidos carboxílicos.
Resultados y conclusionesNo se han encontrado diferencias estadísticamente significativas de los VOC con respecto a la histología.
Los pacientes con CPNM con EPOC presentan 1,7 (1,1–2,7) veces más probabilidad de detección de ácido propanoico (IC 95%: 1,22−6,2) que los pacientes CPNM sin EPOC (p = 0,013).
The etiology of lung cancer (LC), like any other neoplasm, is multifactorial. However, the hereditary genetic component in LC is minor compared to the role played by carcinogens in tobacco smoke.1 This would explain why LC is often associated with chronic obstructive pulmonary disease (COPD), which is also closely linked to smoking. Other factors, such as exposure to diverse substances, environmental pollution, and radiation,2,3 also determine multiple genetic mutations (EGFR, EML4-ALK fusion protein which is especially important in adenocarcinomas,4,5 p53 alterations, and many others6).
In several studies,7,8 COPD is considered a risk factor for LC, but this, on the other hand, begs the question why, in populations with similar etiological risk factors, a large group of COPD patients do not develop LC. Generally, when cases do overlap, COPD precedes LC, which is logical when the pathogenesis of both diseases is taken into consideration.
The strain of non-small cell lung cancer (NSCLC) most closely linked to tobacco use is squamous cell carcinoma, while smoking plays a more minor role in adenocarcinoma.9,10 Over the past few decades, the incidence of adenocarcinomas compared to squamous cell carcinoma has increased at rates that vary from country to country.11 Some authors conclude that women are more vulnerable to the harmful effects of smoking,12 possibly due to the role of estrogens in tumor growth.13
Exposure to tobacco smoke and other environmental substances triggers sustained inflammation due to increased oxidative stress, a phenomenon that is common to both diseases. One example of the effects of oxidative stress is lipid peroxidation,14 caused by the action of free radicals or reactive oxygen species on polyunsaturated fatty acids that form part of cell membranes. Disrupting these molecules generates low molecular weight substances called volatile organic compounds (VOCs) that are excreted during breathing. VOC metabolomics provide an indirect measure of the level of oxidative stress.
The first relevant studies that differentiated patients with LC using highly specific and sensitive VOC patterns were published by Philips et al.15,16 and Poli et al.17 These studies were highly criticized for not having demonstrated the biological origin of the VOCs, but notwithstanding, they opened up a line of research based on the determination of alkanes.
In 2010, Fuch et al.18 and Poli et al.19 published studies investigating VOC in LC, focusing on the determination of aldehydes as biomarkers, given their known association with lipid peroxidation.
In 2017, Callol-Sanchez et al.,20 in a study that included a healthy population, a COPD group, and an LC group, analyzed aldehydes (hexanal, heptanal, and nonanal) and carboxylic acids (propanoic and nonanoic) in exhaled air, a novel approach at the time. They concluded that nonanoic acid differentiated between the LC group and the healthy and COPD groups.
In view of these data, we propose that the coexistence of LC with COPD will produce a characteristic VOC pattern.
The aim of this study was to determine the relative influence of COPD on the VOC profile of patients with NSCLC (squamous cell, adenocarcinoma, and large cell undifferentiated carcinoma).
Patients and methodsThis was a cross-sectional study with consecutive non-probabilistic sampling. In total, 107 individuals were selected and divided into 2 groups: NSCLC with COPD (n = 67) and NSCLC without COPD (n = 40).
All patients were recruited from the Hospital Central de la Defensa Gómez Ulla and the Hospital Clínico San Carlos in Madrid.
The data collection period was from October 2010 to December 2017.
The inclusion criteria for both groups were: informed consent, age over 40 years old, and never smokers, former smokers or active smokers, according to World Health Organization criteria. There was no gender limitation.
All patients underwent protocolized clinical and laboratory diagnostic tests for the diagnosis and stratification of LC and COPD (GOLD).21
The existence of active or previous malignancy in any system or organ was considered an exclusion criterion.
All participants were informed about the study objectives, risks, benefits, tests, and techniques. Data were collected subject to the provisions of the Organic Data Protection Law 15/1999, of 13 December, and Law 41/2002 Regulating Patient Autonomy and Health Documentation and Information-Related Rights and Obligations, of 14 November. The study protocol was approved by the Ethics and Clinical Research Committee of the Hospital Central de la Defensa Gómez Ulla (No. R: IS-404030-S-06-000153).
Samples of 900 ml of exhaled air were obtained for analysis from all patients in 3 forced expiratory maneuvers using a BioVOC device, and the air was transferred to thermal desorption tubes. The same amount of room air was collected from the area where the study subject performed the test to compare VOC levels in room and exhaled air. Samples were analyzed using thermal desorption-gas chromatography and mass spectrometry within 24 h of collection to minimize reactions between compounds and their oxidation. A compound was defined as endogenous when it was present in exhaled air and not in room air or, if it was present in both, when the exhaled/room air ratio was greater than 1. This protocol has been described in previous publications by our group.19,22,23
The compounds studied were: hexanal, heptanal, nonanal, propanoic acid, and nonanoic acid.
In the statistical study, independent variables were assessed: age, sex, smoker, former smoker, never smoker, histology, individuals with NSCLC with COPD, and individuals with NSCLC without COPD. Dependent qualitative variables were those derived from the determinations of the following VOCs: aldehydes and carboxylic acids.
After verification of normality (Kolmogorov–Smirnov) of the distributions of the different VOCs in exhaled air, the arithmetic mean and standard deviation or median and interquartile range for non-parametric distributions were used for central tendency measures and dispersion for parametric distributions.
Associations between dichotomous independent and quantitative dependent variables were verified using the Mann–Whitney U test Effect and precision were verified by the difference in medians and their corresponding 95% CI.
The association between independent and dependent categorical variables was verified by Pearson’s χ2 test. Risk estimation was made using prevalence ratios and their corresponding 95% CI.
A p value of < 0.05 was considered a statistically significant association for all statistical analyses.
The statistical program SPSS® v. 24. (SPSS Inc. Chicago, IL, USA).
ResultsOur population consisted of 107 patients with a mean age (±SD) of 68 (±11.4) years, 77% men with a mean age of 70 (±10) years, and 23% women aged 65 (±13).
Overall, 86% were smokers or former smokers, with a median pack-year index (PYI) of 59 and interquartile range (IQR) of 59. A total of 87% were men with a median PYI of 59 (IQR: 59) and 23% women with a PYI of 35 (IQR: 34). This difference in medians is statistically significant (p = 0.039).
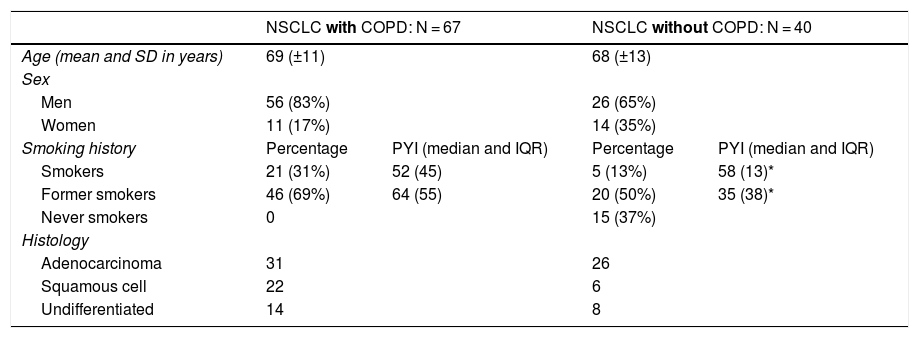
The population was divided into 2 groups (67 with NSCLC with COPD and 40 with NSCLC without COPD). The most prevalent non-small-cell histologies, adenocarcinoma, squamous cell, and large cell undifferentiated, were selected. Patient characteristics are listed in Table 1.
Demographic characteristics of study population.
| NSCLC with COPD: N = 67 | NSCLC without COPD: N = 40 | |||
|---|---|---|---|---|
| Age (mean and SD in years) | 69 (±11) | 68 (±13) | ||
| Sex | ||||
| Men | 56 (83%) | 26 (65%) | ||
| Women | 11 (17%) | 14 (35%) | ||
| Smoking history | Percentage | PYI (median and IQR) | Percentage | PYI (median and IQR) |
| Smokers | 21 (31%) | 52 (45) | 5 (13%) | 58 (13)* |
| Former smokers | 46 (69%) | 64 (55) | 20 (50%) | 35 (38)* |
| Never smokers | 0 | 15 (37%) | ||
| Histology | ||||
| Adenocarcinoma | 31 | 26 | ||
| Squamous cell | 22 | 6 | ||
| Undifferentiated | 14 | 8 | ||
The NSCLC group with COPD consisted of 67 patients, with a mean age of 69 (± 11) years, 56 of whom were men (83%) and 11 women (17%). Overall, the group comprised active smokers with a median PYI of 52 (IQR 45) or former smokers with an PYI of 64 (IQR: 55).
The NSCLC group without COPD consisted of 40 patients, with a mean age of 68 (± 13) years, 26 of whom were men (65%) and 14 women (35%); 13% were active smokers with a PYI of 58 (IQR: 13), while 50% were former smokers with a PYI of 35 (IQR: 38).
In all cases, COPD was 1.6 (1–2.5) times more prevalent in men than women (p = 0.028).
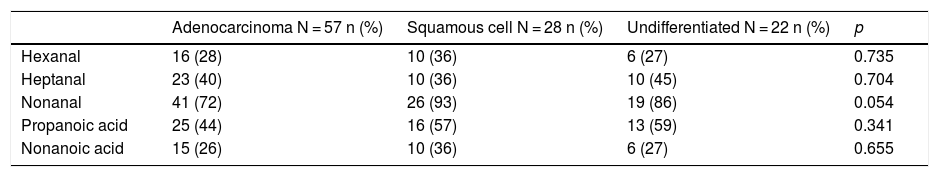
The comparative study of VOCs according to histology is shown in Table 2, which presents the number of cases and the rate of detection of each of the VOCs studied, comparing the 3 histological groups, and p values based on the Pearson χ2 test.
Detection of VOC in different histological strains of NSCLC.
| Adenocarcinoma N = 57 n (%) | Squamous cell N = 28 n (%) | Undifferentiated N = 22 n (%) | p | |
|---|---|---|---|---|
| Hexanal | 16 (28) | 10 (36) | 6 (27) | 0.735 |
| Heptanal | 23 (40) | 10 (36) | 10 (45) | 0.704 |
| Nonanal | 41 (72) | 26 (93) | 19 (86) | 0.054 |
| Propanoic acid | 25 (44) | 16 (57) | 13 (59) | 0.341 |
| Nonanoic acid | 15 (26) | 10 (36) | 6 (27) | 0.655 |
p Value based on Pearson's χ2 test.
The data obtained in our series showed no statistically significant differences in VOCs associated with histology, possibly due to the size of our series.
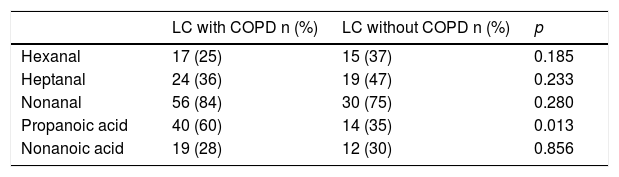
The comparative study of VOCs in the NSCLC with COPD and NSCLC without COPD groups is shown in Table 3.
Influence of COPD on the determination of VOC in patients with NSCLC with COPD and without COPD.
| LC with COPD n (%) | LC without COPD n (%) | p | |
|---|---|---|---|
| Hexanal | 17 (25) | 15 (37) | 0.185 |
| Heptanal | 24 (36) | 19 (47) | 0.233 |
| Nonanal | 56 (84) | 30 (75) | 0.280 |
| Propanoic acid | 40 (60) | 14 (35) | 0.013 |
| Nonanoic acid | 19 (28) | 12 (30) | 0.856 |
p Value based on Pearson's χ2 test.
Statistically significant differences were found in the detection of propanoic acid only. Propanoic acid is 2.7 (1.1–1.7) times more likely to occur in NSCLC patients with COPD (95% CI: 1.22–6.2) than in NSCLC patients without COPD (p = 0.013).
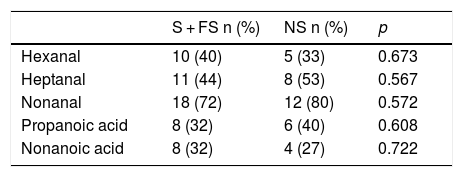
The NSCLC without COPD subgroup consisted of both smokers (smokers and former smokers) and never smokers. Even though the groups are very small, we studied the influence of smoking in this subgroup (Table 4), but failed to find any influence of smoking on the detection of VOCs.
Detection of VOCs in patients with LC without COPD by smoking habit.
| S + FS n (%) | NS n (%) | p | |
|---|---|---|---|
| Hexanal | 10 (40) | 5 (33) | 0.673 |
| Heptanal | 11 (44) | 8 (53) | 0.567 |
| Nonanal | 18 (72) | 12 (80) | 0.572 |
| Propanoic acid | 8 (32) | 6 (40) | 0.608 |
| Nonanoic acid | 8 (32) | 4 (27) | 0.722 |
FS: former smoker; NS: never smoker; S: smoker.
The paper aimed to establish differences between exhaled VOCs in a NSCLC population with and without COPD.
We focused on non-small-cell tumors which have important differences in terms of pathology and chronicity, and on VOC determinations described in previous papers.19,20,22,23 The numerous studies on the determination of VOC in LC have shown wide variation in populations, sample collection and processing, methodology, and the search and identification of possible markers.24,25
Our study focused on aldehydes and carboxylic acids, as these 2 groups of compounds were frequently detected in previous studies.19,20,22,23 Priority was given to these compounds because their origin as final metabolites of fatty acid lipid peroxidation in cell membranes and oxidative metabolism of glucose is well established. In our studies, we assess the interference of environmental VOCs and problems with the sample collection.20,22,23
As COPD and cancers are both the cause and the result of oxidative processes, the coexistence of both diseases may be important in the detection of VOC.
We first studied possible differences in VOC in exhaled air according to the most prevalent histology (Table 2), and found no statistically significant differences between the 3 groups (adenocarcinoma, squamous cell, and large cell undifferentiated). The p value of 0.054 for nonanal was close to statistical significance, so this parameter should be reevaluated in larger numbers of squamous cell and undifferentiated types. The results are consistent with the proposal that VOCs originate from lipid peroxidation26 of tumor cells, as mentioned above. This appears to be a common process not affected by histology, in contrast to findings from other authors,27 who, using similar techniques, established VOC profiles specific to certain LC cell lines.28 However, the VOCs determined in those studies (benzaldehyde, 2-ethylhexanol and 2,4-decadien-1-ol) are of uncertain metabolic origin, and this choice has been criticized in other publications.20,22,23,29
Secondly, VOCs were determined in groups of patients with NSCLC with COPD and NSCLC without COPD.
LC is a variable that influences the determination of nonanoic acid,20 indicating its importance as a lipid peroxidation factor. We also know that hexanal is more frequently detected in COPD.23 On the other hand, a current or former smoking habit affects the presence of nonanal, another VOC generated by lipid peroxidation, which persists for long periods after smoking cessation.22
It is still unclear how these variables (LC, COPD and smoking) interconnect. In patients with NSCLC with COPD and patients with NSCLC without COPD, the presence of propanoic acid was statistically significant (p = 0.013). We have not found this finding reflected in any publication in which similar groups have been studied.
Although propanoic acid derives from both the degradation of membrane fatty acids and the oxidation of glycolysis metabolites (glycerol and glyceraldehyde), its significance in the development of malignant disease or COPD has not yet been clarified.
The NSCLC without COPD subgroup is heterogeneous in terms of smoking. Smokers without COPD show a significant difference in PYI values compared to smokers with COPD (p = 0.035). However, no relationship was detected between VOC determinations and smoking in this subgroup.
This finding appears to contradict the results of a study in smoking in a healthy population,22 but the influence of the LC variable must be taken into account. We can conclude that smoking contributes to oxidative stress less than cancer.
In summary, tumor histological type does not appear to influence the determination of exhaled VOC, but there is an association with propanoic acid in NSCLC patients with COPD. Larger studies need to be conducted before these findings can be applied in the clinic.
FundingThis study was funded by the Carlos III Institute of Health (PI07/1116), SEPAR 2013 (registry no.° 135).
Conflict of interestsThe authors state that they have no conflict of interests.
Please cite this article as: Mu˜noz-Lucas MÁ, Jareño-Esteban J, Gutiérrez-Ortega C, López-Guijarro P, Collado-Yurrita L, Quintana-Díaz M, et al. Influencia de la enfermedad pulmonar obstructiva crónica en los componentes orgánicos volátiles en pacientes con cáncer de pulmón no microcítico. Arch Bronconeumol. 2020. https://doi.org/10.1016/j.arbres.2019.12.023