The inflammatory myofibroblastic tumor (IMT) is a mesenchymal lesion that can arise in multiple locations that have been described in the literature, predominantly in the retroperitoneum and abdomino-pelvic region, including mesentery, omentum, urinary bladder, spleen, liver or even the breast, bladder and larynx. In the WHO classification it is considered a tumor of potential intermediate malignancy. It appears under different names such as inflammatory pseudotumor, plasma cell granuloma or histiocytoma. Currently, this terminology is not recommended by the WHO. Pulmonary involvement was first described in 1973.1 It is a rare cause of primary lung tumor in adults; however, it is the most frequent cause of lung tumor in children, which, according to the series of Hartman et al.2 corresponds to as many as 56% of benign lung tumors at this age. The pathogenesis of this lesion is controversial, having been formerly considered benign lesions that originated as an exaggerated local inflammatory response against tissue damage. However, the discovery of the presence of anaplastic lymphoma kinase gene (ALK gene) rearrangements in about 50% of these lesions gives them a likely neoplastic origin. The recent development of ALK inhibitors therapies could be an alternative treatment.3
A twenty-seven year-old male presented a pulmonary nodule in the right upper lobe in a chest-X-ray done as a pre-operative examination due to a spinal disc herniation. He was asymptomatic, the physical examination was unremarkable and the blood analysis as well as arterial blood gas and pulmonary function tests showed no alterations. The chest computed tomography (CT) reported the presence of a solid, homogenous mass of 42mm in diameter in the right upper lobe. No significant alterations in lung parenchyma or the presence of pathological size adenopathies were observed. A bronchoscopy was carried out and found a well-defined hipervascular mass occluding the posterior segmental bronchus of the right upper lobe. Transbronchial needle aspiration of the lesion was performed.
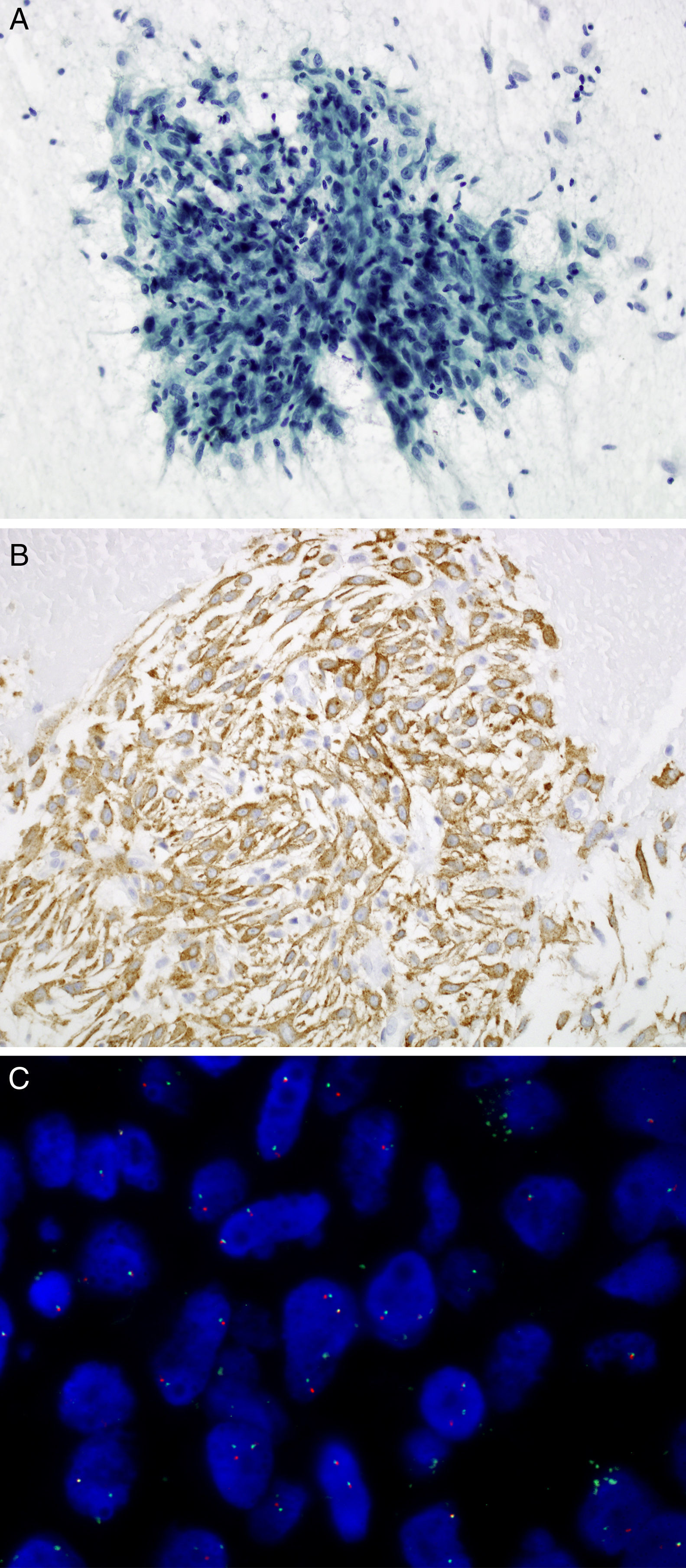
Cytological study of the smears showed densely cellular extensions constituted by groups of spindle cells, loosely cohesive in some parts. Cells presented elongated nuclei, with mild cytological atypia. Admixed with these cells was a mild infiltrate of lymphoplasmacytic cells (Fig. 1A), along with foamy macrophage and areas of myxoid appearance. Figures of mitosis or areas of necrosis (Fig. 1A) were not identified. An immunohistochemical study of the cell block was made and the spindle cells showed an intense cytoplasmic expression of smooth muscle actin, vimentin and weak expression of desmin in isolated cells. On the other hand, the expression of cytokeratins AE1-AE3, protein S100, CD34 and CD68 (expression in macrophages) was not demonstrated in tumor cells. The proliferation index by Ki67 was 2%. Immunohistochemical analysis showed positive staining for ALK D5F3 (Fig. 1B). The rearrangement of this gene was confirmed by FISH (Fig. 1C). Based on these data, the IMT diagnosis was made.
(A) Transbronchial FNA: spindle cells admixed with lymphoplasmacytic cells. Papanicolaou, 20×. (B) Cell block: tumor cells show cytoplasmic expression of the protein ALK using D5F3 antibody. 20×. (C) ALK FISH break-apart probe (Vysis, Abbott Molecular) showing a typical rearranged positive pattern with mainly one fused and one split signals (1F1O1G).
A lobectomy specimen obtained 3 months later showed a mesenchimal tumor with spindle cells and abundant inflammatory infiltrate of lymphoplasmacytic cells and histiocytes. The immunohistochemical studies showed the same pattern as detected in the cytological cell block and we could also detect the ALK fusion gene by FISH.
IMT of the lung is a rare benign lesion. Its frequency is estimated between 0.04% and 0.7%4 of all primary lung tumors in adults. It can occur in any age group, but about 50% of patients are under 40 years of age, mostly children. The male-to-female ratio is about 1:1. Pathogenesis of IMT is not known. An unregulated proliferation of inflammatory cells due to an initial infection or an autoimmune disease was a favored hypothesis. However, recently, the discovery that up to 50% of these lesions have ALK gene rearrangement suggests that it might have a true neoplastic origin. This rearrangement leads to constitutive activation and overexpression of ALK causing cell proliferation. ALK protein expression by immunohistochemistry correlates with the detection of this gene alteration by FISH.5 Some fusion proteins of this gene have been described in pulmonary IMTs such as tropomyosin3-anaplastic lymphoma kinase (TPM3-ALK), protein tyrosine phosphatase receptor-type F polypeptide-interacting protein binding protein 1-anaplastic lymphoma kinase (PPFIBPIALK), and even echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK), the latter considered largely specific to lung adenocarcinoma.6
Although most of the patients are asymptomatic, they can present cough, chest pain, dyspnea, and even hemoptysis as well as systemic symptoms such as fever, weight loss and fatigue. The blood test can sometimes show anemia among others. Radiologically, 87% of cases occur as well-defined nodules or masses only,7 rarely multiple (5%). The presence of calcification is detected in less than 15% of the cases and cavitation is very rare. Macroscopically they are grayish well-defined, non-encapsulated tumors. Microscopically, the lesion presents an inflammatory infiltrate of plasma cells, lymphocytes, and histiocytes with a variable proportion of fibroblasts and myofibroblasts, so the differential diagnosis should be done with all the entities that present a polymorphous cell population with mild atypia like low grade sarcoma, lymphoma or spindle cell tumors. Three histologic subtypes depending on the cell population that predominate have been described.8 The treatment is complete surgical resection with negative resection margins. After surgical resection, between 10 and 25% of patients may experience local recurrence but the risk of metastasis is low (<5%). Recently it has been described that this risk is related to the presence of ALK rearrangement, being lower in patients with this genetic alteration. However, the presence of this genetic alteration is not related to the risk of local recurrence.9 In cases where surgical treatment is contraindicated, such as multifocal lesions, local tumor invasion or recurrence, radiotherapy and/or chemotherapy can be useful. Currently, the development of therapies targeting tyrosine-kinase receptor has meant a new therapeutic target there are cases described with radiological responses in this type of tumors.3
Finally, in this case we highlight the importance of TBNA for the diagnosis of this entity, avoiding more aggressive diagnostic procedures, as well as the possibility of performing FISH techniques on the retrieved material.