The effect of hereditary alpha-1 antitrypsin (AAT) deficiency can manifest clinically in the form of chronic obstructive pulmonary disease (COPD). AAT deficiency (AATD) is defined as a serum concentration lower than 35% of the expected mean value or 50mg/dl (determined by nephelometry). It is associated in over 95% of cases with Pi*ZZ genotypes, and much less frequently with other genotypes resulting from combinations of Z, S, rare and null alleles. A systematic qualitative review was made of 107 articles, focusing mainly on an active search for AATD in COPD patients and intravenous (iv) treatment with AAT. On the basis of this review, the consultant committee of the Spanish Registry of Patients with AATD recommends that all COPD patients be screened for AATD with the determination of AAT serum concentrations, and when these are low, the evaluation must be completed with phenotyping and, on occasions, genotyping. Patients with severe AATD COPD should receive both the pharmacological and non-pharmacological treatments recommended in the COPD guidelines. There is enough evidence from large observational studies and randomized placebo-controlled clinical trials to show that the administration of iv AAT reduces mortality and slows the progression of emphysema, hence its indication in selected cases that meet the inclusion criteria stipulated in international guidelines.
The administration of periodic infusions of AAT is the only specific treatment for delaying the progression of emphysema associated with AATD.
El déficit hereditario de la alfa-1 antitripsina (AAT) se puede manifestar clínicamente como una enfermedad pulmonar obstructiva crónica (EPOC). Se define por una concentración sérica por debajo del 35% del valor medio esperado, o 50mg/dL (medida por nefelometría) y está relacionado en más del 95% de los casos, con genotipos Pi*ZZ, y muy infrecuentemente con otros genotipos resultantes de combinaciones entre alelos Z, S, raros y nulos. Se ha realizado una revisión sistemática cualitativa de 107 artículos, centrados principalmente en la búsqueda activa del déficit de AAT (DAAT) en pacientes con EPOC y en el tratamiento con AAT por vía intravenosa (iv). El comité asesor del Registro Español de pacientes con DAAT, sobre la base de esta revisión, considera que se debe descartar el DAAT, mediante la cuantificación de las concentraciones séricas de AAT, en todos los pacientes con EPOC y cuando sean bajas se debe completar el estudio mediante la determinación del fenotipo y, en ocasiones, del genotipo. El tratamiento de los individuos con EPOC asociado a DAAT grave debe incluir el tratamiento farmacológico y no farmacológico recomendado en las normativas de la EPOC. Existe suficiente evidencia, derivada de grandes estudios observacionales y de ensayos clínicos aleatorizados con placebo, que demuestran que el tratamiento con AAT iv disminuye la mortalidad y reduce la velocidad de progresión del enfisema, por lo que está indicado en casos seleccionados que cumplan los criterios de inclusión establecidos en las normativas internacionales.
La terapia con infusiones iv periódicas de AAT es el único tratamiento específico que existe para frenar la progresión del enfisema asociado al DAAT.
Hereditary alpha-1 antitrypsin (AAT) deficiency can manifest clinically in the form of chronic obstructive pulmonary disease (COPD) (typically as panacinar pulmonary emphysema), liver cirrhosis at any age and, less commonly, as panniculitis, systemic vasculitis and other diseases.1 Severe AAT deficiency (AATD) is defined as a serum AAT level lower than 35% of the expected mean value or less than 50mg/dl (determined by nephelometry). It is associated with Pi*ZZ genotypes in over 95% of cases, and much less frequently with other genotypes resulting from combinations of Z, S, rare and null alleles.2
Since the detection of severe AATD cases involves genetic counseling, the study of first-degree relatives and, in selected cases, the administration of regular intravenous (IV) AAT infusions, in 2006, the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), in collaboration with the Spanish Registry of Patients with AATD (REDAAT) advisory committee, published guidelines on the diagnosis and treatment of AATD, the basic concepts of which remain valid today.3 However, several subsequent studies4–7 have provided new data supporting the importance of detecting AATD in individuals with COPD and the use of replacement therapy in patients with COPD and severe AATD,8–13 all of which justify this update.
MethodologyThe authors performed a literature search of articles published between 1985 and 2013 in the MEDLINE, EMBASE and Cochrane Library databases, using the keywords: “alpha-1 antitrypsin deficiency”, “COPD”, “asthma”, “bronchiectasis”, “augmentation therapy” and “replacement therapy”. Meta-analyses and systematic reviews by other authors, based on quality of scientific evidence, as well as some articles cited in those selected previously and not detected in the databases, were also included for analysis.
A total of 289 abstracts were obtained using the terms “alpha-1 antitrypsin deficiency” and “COPD”; 154 using “alpha-1 antitrypsin deficiency” and “asthma”; 87 using “alpha-1 antitrypsin deficiency” and “bronchiectasis”; 129 using “alpha-1 antitrypsin deficiency” and “augmentation therapy”; and 71 using “alpha-1 antitrypsin deficiency” and “replacement therapy”.
After 3 general meetings and 1 final single-subject meeting, we performed a qualitative systematic analysis of the articles selected in order to draw up this document. After deleting duplicates found in the various searches, and using the information provided in the abstract of the articles selected (or the full text when this was not sufficiently explicit), 107 articles1–107 were chosen by consensus. Most were focused on the active search for AATD in COPD patients and on replacement therapy in patients with severe AATD-associated COPD. The authors individually assessed the manuscripts considered potentially useful, and rated them according to GRADE System criteria for grading the quality of evidence and strength of recommendations, Regulations for Writing SEPAR Guidelines108,109 and American College of Chest Physicians Task Force110 criteria, amended by the Canadian Thoracic Society COPD Clinical Assembly Alpha-1 Antitrypsin Deficiency Expert Working Group.13 After reviewing the results, the conclusions described below were agreed among the members of the advisory committee.
ResultsThe results of the qualitative systematic analysis are summarized in Tables 1 and 2. It should be noted that the REDAAT working group detected major shortcomings in the literature, which highlight the need for future high quality studies to address several of the issues raised. Even so, analysis of the 4 papers selected49,54,71,93 and a recent high-quality meta-analysis13 focusing on research of AATD in COPD suggest that AATD should be ruled out by measuring serum AAT concentrations in all patients with COPD, and when these are low, the study should be completed by phenotyping and, occasionally, genotyping (consistent recommendation with high quality evidence, which confirms the recommendations proposed in the 2006 guidelines).3
Summary of REDAAT Recommendations on ATTD Screening in COPD, Bronchiectasis and Bronchial Asthma, and on the Use of Replacement Therapy.
| Strength of recommendation | REDAAT recommendations | Quality of evidence |
|---|---|---|
| Consistent recommendation | The working group recommends determination of plasma AAT concentrations in all subjects with COPDa | High quality evidence |
| Consistent recommendation | This working group does not recommend routine determination of AAT concentrations in patients with bronchiectasis. Testing should be on a case-by-case basisb | Low quality evidence |
| Consistent recommendation | This working group does not recommend routine determination of AAT concentrations in asthmatic patients. Testing should be on a case-by-case basisb | Low quality evidence |
| Consistent recommendation | IV AAT replacement therapy is indicated in patients who are never-smokers or former smokers with COPD associated with severe AAT deficiencyd, whose FEV1 is less than 80% of predicted, and who are correctly treated (pharmacological and non-pharmacological treatment of COPD), in whom respiratory functional decline and/or emphysema progression has been documentedc | Moderate quality evidence |
AAT: alpha-1 antitrypsin; IV: intravenous; AATD: alpha-1 antitrypsin deficiency; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second; REDAAT: Spanish Registry of Patients with Alpha-1 Antitrypsin Deficiency.
Consistent recommendation, high quality evidence. Can be applied to most patients in most circumstances.
Severe deficiency is defined as serum AAT concentrations ≤50mg/dl, measured by nephelometry. It is generally associated with PIZZ phenotypes and combinations of rare and null alleles, with each other or with Z and S. AATD is not considered severe when it is associated with the MZ phenotypes or most SZ phenotypes, except for those with AAT concentrations ≤50mg/dl.
Summary of Studies Evaluating Augmentation Therapy.
| Authors | Dose | Type of study | Results measure | Results | Level of evidence |
|---|---|---|---|---|---|
| Non-randomized studies | |||||
| Seersholm et al.37 (1997) | 60mg/kg/7days | Observational study with control group(n=295) | FEV1 decline | Reduction in FEV1 decline in the treatment group (56 vs 75ml/year; P=.02).Greater benefit in patients with FEV1 31%–65% | C2 |
| American AAT Deficiency RegistryStudy Group40 (1998) | 33% weekly doses; 43% fortnightly and 24% monthly | Observational study with control group(n=1129) | FEV1 declineMortality | Reduction in mortality (OR=0.64; P=.02)The FEV1 decline is slower in patients receiving IV replacement therapy with FEV1 35%–49% (66 vs 93ml/year; P=.03) | C2 |
| Wencker et al.53 (2001) | 60mg/kg/7days | Observational cohortwith no control group(n=96) | FEV1 decline | The rate of FEV1 decline was slower during the treatment period (49.2 vs 34.2ml/year, P=.019) and was slower in patients with FEV1>65% (256 vs 53ml/year, P=.001) | C2 |
| Tonelli et al.83 (2009) | Observational study with control group(n=164) | FEV1 declineMortality | Gain in FEV1 of 10.6±21.4ml/year vs loss of 36.96±12.1ml/year; P=.05)No differences in mortality | C2 | |
| Ma et al.42 (2013) | 60mg/kg/7 days | Observational cohort study with control group(n=100) | Plasma desmosine and isodesmosine | A significant reduction in desmosine and isodesmosine levels in the patient cohort on IV AAT replacement therapy vs untreated patients (P<.0001), with values similar to the normal population | C1 |
| Ma et al.42 (2013) | 60mg/kg/7days | Observational studywith no control group(n=10) | Desmosine and isodesmosine in bronchoalveolar lavage and plasma | Significant reduction in desmosine and isodesmosine levels in bronchoalveolar lavage (P=.0273) and in plasma at 12 (P=.0038) and 24 weeks (0.0038) after receiving IV replacement therapy | C2 |
| Randomized studies | |||||
| Dirksen et al.4 (2009) | 60mg/kg/7 days | Randomized, double-blind, placebo-controlled study(n=77) (COPD with FEV1=25%–80%) | Lung function, quality of life, exacerbations and loss of CT lung density | Reduction in loss of CT lung density in treated patients (P=.049)No differences in FEV1 or DLCONo differences in frequency of exacerbations, but less severe in the treatment group | B1 |
| Meta-analyses | |||||
| Chapman et al.12 (2009) | Meta-analysis of studies in patients on replacement therapy vs controls in the Canadian Registry (n=1509) | FEV1 decline | Reduction in FEV1 decline in patients on IV replacement therapy by 26% (17.9ml/year). Effect due to subgroup of subjects with FEV1 30%–65% | B1 | |
| Gøtzsche and Johansen10 (2010) | 60mg/kg/7days | Cochrane meta-analysis of 2 randomized, placebo-controlled studies(n=140) | Decline in FEV1, DLCO and loss of CT lung densityExacerbations | The lung density loss was less in patients on IV replacement therapy (P=.03)No differences in lung functionNo difference in exacerbations | B2 |
| Stockley et al.5 (2010) | 60mg/kg/7 days | Comprehensive analysis of lung density(n=119) | Decline in lung density and FEV1 | Less lung density loss in treated patients (1.73 vs 2.74g/l, P=.006)No differences in FEV1 decline | A1 |
| Marciniuk et al.13 (2012) | Meta-analysis of all studies in patients on IV replacement therapy vs controls | All parameters | Reduction in CT lung density loss and reduction in mortality | B1 | |
| Studies in exacerbations | |||||
| Lieberman46 (2000) | 55% weekly doses, 37% fortnightly and 8% monthly | Observational (internet survey)(n=89) | Frequency of exacerbations | Reduction in the frequency of exacerbations from 3–5/year to 0–1/year after starting IV replacement therapy | C2 |
| Stockley et al.55 (2002) | 60mg/kg/7days | Descriptive study(n=12) | Inflammatory markers in sputum | Significant reduction in LTB4 in sputum following treatment | C2 |
| Barros-Tizón et al.96 (2012) | 180mg/kg/21days | Retrospective study (pre–post IV replacement therapy) | Frequency and severity of exacerbations and hospitalization costs | Reduction in the number and severity of exacerbations and hospitalization-derived costs | C1 |
AAT: alpha-1 antitrypsin; IV: intravenous; AATD: alpha-1 antitrypsin deficiency; DLCO: carbon monoxide diffusing capacity; FEV1: forced expiratory volume in the first second; LTB4: leukotriene B4; CT: computed tomography.
The working group, based on the level of evidence provided by 13 specific studies on IV AAT treatment,4,5,10,12,13,37,40,42,46,53,55,83,96 considers that replacement therapy is indicated in patients with severe AATD (defined as an AAT concentration ≤50mg/dl, measured by nephelometry), never-smokers or former smokers, diagnosed with COPD and lung function decline (FEV1<80% of predicted value), with documented loss of lung function or emphysema progression, despite optimal pharmacological and non-pharmacological treatment of COPD. Replacement therapy is not indicated in Pi*MZ heterozygotes or in most Pi*SZ individuals, except in rare cases of SZ heterozygotes with serum AAT concentrations less than or equal to 50mg/dl who meet the other criteria listed in Tables 3 and 4 (consistent recommendation with moderate quality evidence, and in accordance with SEPAR guidelines).3 Replacement therapy is not indicated for liver disease caused by AATD.
REDAAT Criteria for IV AAT Therapy.a
| 1. Aged 18 years or over |
| 2. AATD demonstrated by serum concentrations ≤50mg/dl |
| 3. Never-smokers or ex-smokers for at least the last 6 months |
| 4. Pulmonary emphysema demonstrated by lung function tests and/or chest HRCT |
| 5. COPD with FEV1 <80% of predicted,b who are receiving optimal pharmacological and non-pharmacological treatment |
| 6. Do not have immunoglobulin A deficiency |
| 7. Prepared to receive regular treatment at a day hospital |
AAT: alpha-1 antitrypsin; IV: intravenous; AATD: alpha-1 antitrypsin deficiency; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second; REDAAT: high resolution computed tomography.
Procedure to be Followed Prior to Commencing IV AAT Replacement Therapy.
| Informed consenta |
| Additional tests |
| Determination of serum immunoglobulins |
| Complete liver function tests |
| Serology for hepatitis-B virus and human immunodeficiency virus |
| Lung function tests: spirometry, lung volumes and carbon monoxide diffusing capacity |
| Arterial blood gases: if peripheral oxygen saturation is less than 92% |
| Chest HRCT scan |
| Hepatitis B vaccination |
AAT: alpha-1 antitrypsin; IV: intravenous; HRCT: high resolution computed tomography.
Available on the website of the (http://www.redaat.es/presentacion.php) and the Andalusia Regional Government Health website, in the area on Informed Consent for Respiratory Medicine procedures (http://www.juntadeandalucia.es/salud/sites/csalud/contenidos/Informacion_General/p_3_p_11_procedimiento_consentimiento_informado/neumologia?perfil=org).
With regard to the results of 3 studies6,33,48 on the prevalence of AATD in patients with bronchiectasis and 1 meta-analysis,13 the REDAAT working group does not recommend routine determination of AAT concentrations in patients with bronchiectasis. This practice should be determined on a case-by-case basis (consistent recommendation with low quality evidence).
Considering the results of 5 studies on the prevalence of AATD in asthmatic patients7,49,54,56,70 and 1 meta-analysis,13 the authors do not recommend routine measurement of AAT concentrations in these patients. This practice should be determined on a case-by-case basis (consistent recommendation with low quality evidence).
DiscussionThe results shown support the recommendation to rule out AATD in all patients with COPD. This was proposed by the World Health Organization as far back as 1997,35 and was subsequently included in various guidelines, including those of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and SEPAR.1,3 Furthermore, although there are insufficient studies to precisely establish a strength of recommendation, the authors also advise ruling out AATD in first-degree relatives of the index case, even if they are asymptomatic, due to the high probability that some may be carriers of severe mutations and may benefit from genetic counseling and preventive measures (the most important being to avoid inhaling cigarette smoke and other pollutants).1,3
With respect to other obstructive airways diseases, the REDAAT working group agrees with other authors13 in not recommending routine determination of AAT levels to rule out severe deficiency in patients with either bronchiectasis or asthma, leaving the decision to request this test in specific cases at the discretion of the attending physician, e.g. in patients with emphysema lesions associated with the aforementioned conditions.
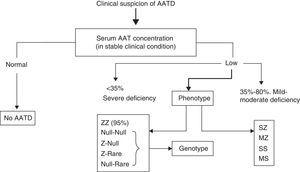
Quantitative serum AAT measurement, most often by nephelometry, is the basis for diagnosing AATD. When the AAT concentration is lower than the reference range, the study should be completed by phenotyping (protein or allele variants). The combination of both techniques is sufficient to clarify most cases of AATD. Isoelectric focusing is the most widely used method to identify allelic variants, and can characterize up to 30 AAT deficiency variants.
Since each phenotype has its own range of AAT values, in cases in which the AAT concentration is not consistent with the phenotype, the presence of null or rare deficient alleles should be suspected, and consequently, the genotype should be determined.95 AAT gene sequencing by real time polymerase chain reaction (PCR) is the reference method for clarifying these discordant cases (Fig. 1).3,95
The dried blood spot samples used in screening programs indicate the presence or absence of the alleles studied, but cannot exclude the presence of other deficiency alleles. Genotyping must therefore be performed in cases in which the AAT concentration is not consistent with the phenotype. Dried blood spot samples can currently be genotyped using PCR sequencing. These methods are reliable, but each laboratory must report the method used and its possible limitations.
With respect to treatment, the REDAAT working group considers that:
- –
Treatment of individuals with severe AATD-associated COPD should include the pharmacological and non-pharmacological treatment recommended in COPD guidelines.111
- –
There is sufficient evidence available (although of moderate quality4,5,10,12,13,37,40,42,46,53,55,83,96) to recommend replacement therapy in individuals with COPD associated with severe AATD (serum AAT concentrations ≤50mg/dl), never-smokers or former smokers, whose FEV1 is less than 80% of predicted and who have loss of lung function or emphysema progression, despite standard COPD treatment.
- –
Regular AAT replacement therapy is the only specific treatment to slow the progression of AATD-associated emphysema. Its efficacy has been demonstrated in randomized, double-blind, placebo-controlled studies, with analysis of the decline in lung density as the primary outcome measure.
Table 3 specifies the REDAAT requirements for replacement therapy. Table 4 provides more detail on the procedure to be followed before commencing treatment. Table 2 lists the main studies on its efficacy or effectiveness in patients with COPD and severe AAT, and the quality of evidence.
The efficacy of replacement therapy is defined on the basis of biochemical and clinical criteria. Biochemical efficacy has been demonstrated, as IV AAT administration increases serum values above those considered protective, increases its concentration in alveolar fluid, and neutralizes neutrophil elastase.18,19 It has been agreed that the serum AAT value that protects the lung against free neutrophil elastase should be greater than or equal to 50mg/dl (if determined by nephelometry), 80mg/dl (if measured by radial immunodiffusion) or 11μM/l (if the NHLBI standard from the US Registry is used). This widely disseminated and applied laboratory criterion is based on studies by Wewers et al. (1987)18 and Turino et al. (1996).34 The former18 shows the biochemical efficacy of AAT replacement therapy (moderate quality evidence) but is insufficient to establish a protective cut-off value. The latter34 describes the clinical characteristics of 59 SZ subjects, of whom 52% were receiving or had received IV AAT treatment, and has been used to justify the biochemical criteria for patient selection for AAT replacement therapy. However, the quality of scientific evidence is very low, and its findings cannot be extrapolated to the other SZ subjects. Similar arguments are applicable to another descriptive study in 25 SZ subjects.17
Various studies have shown the clinical efficacy of replacement therapy,4,10,12,13,37,40,42,46,53,55,83,96 the most important being the study by Dirksen et al.,4 a randomized, double-blind, placebo-controlled trial in which the primary endpoint was loss of lung density measured by computed tomography (CT). This showed a significantly lower annual loss of lung density in subjects who received AAT replacement therapy compared to those who did not. There were no differences in lung function, exacerbations and quality of life (St. George's questionnaire) between groups. A subsequent analysis by Stockley et al.,5 combining data from these 2 trials, confirmed lung tissue loss was lower in subjects treated with IV AAT vs placebo (P=.006).
A meta-analysis conducted by Chapman et al. on 5 studies with a total of 1509 patients found that AAT replacement therapy significantly reduced annual decline in FEV1, especially in patients with an FEV1 between 30% and 65% of predicted.12
In another meta-analysis, Gøtzsche and Johansen10 concluded that AAT replacement therapy cannot be recommended, based on its lack of efficacy and high cost. However, this analysis has been widely criticized by the scientific community and patient associations, such as the Alpha-One Foundation, for its partiality. It bases its conclusions on 2 studies with a total of 140 patients and downplays the importance of measuring loss of CT lung density, when this loss is a central feature in the natural history of these patients. Furthermore, it excludes the results of some observational studies that support the clinical efficacy of AAT replacement therapy, and which have been the basis for its indication in ATS, ERS and American College of Physicians guidelines, including the multicenter prospective cohort study conducted in 1129 patients with AATD in the American Registry. This study showed a 36% reduction in mortality (Relative Risk=0.64) and a significant reduction in the FEV1 decline in the subgroup of patients with FEV1 values between 35% and 49%.40
Finally, a recent, extremely thorough meta-analysis by the Canadian Thoracic Society13 recommends replacement therapy in patients with COPD and FEV1 between 25% and 80%, never-smokers or former smokers, with documented AATD (11μmol/l), who are receiving optimal pharmacological and non-pharmacological treatment (including rehabilitation), because of the benefits that it provides (less loss of lung density, demonstrated by CT densitometry, and reduction in mortality).
In conclusion, severe AAT deficiency is a rare genetic condition that principally manifests clinically as pulmonary emphysema. There is sufficient evidence to recommend AAT replacement therapy (Table 2) in patients who meet certain conditions (Table 3).
This working group is of the opinion that further studies are warranted to better determine the mechanisms that lead to the development of COPD in subjects with AATD, and to determine, with stronger evidence, the AAT level needed to protect the lung from the elastolytic action of elastase, in stable conditions and in the case of exacerbation, as well as the dose of AAT required to reach these protective levels. Finally, the authors believe that better and more cost-effective means for producing and administering AAT must be found.
FundingThe authors have not received any funding for this article.
Conflict of InterestThe Spanish Lung Foundation (Respira) received donations from Laboratorios Grifols for sponsoring activities by the Spanish Registry for Patients with Alpha-1 Antitrypsin Deficiency.
Ana Bustamante received honoraria for lecturing from Grifols, Astra, Boheringer-Ingelheim, Pfizer, Chiesi, and Almirall.
Francisco Casas received honoraria for scientific advice and/or for lecturing from Almirall, AstraZeneca, Boehringer Ingelheim, Grupo Ferrer, GlaxoSmithKline, Grifols, Laboratorios Esteve, Pfizer, Novartis and Takeda.
José María Hernández received honoraria from Grifols for scientific advice and for lecturing.
Lourdes Lázaro received honoraria from Grifols for lecturing.
Beatriz Lara received honoraria for lecturing from Boehringer Ingelheim, Pfizer, Grifols and Novartis.
Marc Miravitlles received honoraria for scientific advice and/or for lecturing from Almirall, AstraZeneca, Bayer Schering Boehringer Ingelheim, Grupo Ferrer, GlaxoSmithKline, Grifols, Laboratorios Esteve, Pfizer, Novartis and Nycomed.
María Torres received honoraria from Grifols for scientific advice.
The REDAAT Committee would like to thank Doctors Rafael Vidal, Rosendo Jardí, Juan Carlos Barros-Tizón, Pedro Pablo España and Carlos Escudero for their contribution to the work of the Registry over the years.
Please cite this article as: Casas F, Blanco I, Martínez MT, Bustamante A, Miravitlles M, Cadenas S, et al. Actualización sobre indicaciones de búsqueda activa de casos y tratamiento con alfa-1 antitripsina por vía intravenosa en pacientes con enfermedad pulmonar obstructiva crónica asociada a déficit de alfa-1 antitripsina. Arch Bronconeumol. 2015;51:185–192.