Indications for inhaled corticosteroids (IC) in combination with long-acting bronchodilators (LABD) are well defined in clinical practice guidelines. However, there are some doubts about their efficacy and safety. The aim of this document is to establish an expert consensus to clarify these issues.
MethodA coordinator group was formed, which systematically reviewed the scientific evidence with the aim of identifying areas of uncertainty about the efficacy of ICs, the adverse effects associated with their use and criteria for withdrawal. Their proposals were submitted to a panel of experts and the Delphi technique was used to test the level of consensus.
ResultsTwenty-five experts participated in the panel, and consensus was reached on the use of IC in the mixed chronic obstructive pulmonary disease (COPD)-asthma phenotype and in frequent exacerbators, and on not using IC in association with LABD for improving lung function in COPD. There was no general consensus on restricting the use of IC to prevent adverse effects. The panel did agree that IC withdrawal is feasible but should be undertaken gradually, and patients who have discontinued must be evaluated in the short term.
ConclusionsConsensus was reached regarding the indication of IC in mixed COPD-asthma and frequent exacerbator phenotypes. The potential for adverse effects must be taken into consideration, but there is no consensus on whether limiting use is justified. The withdrawal of ICs was uniformly agreed to be feasible.
Las indicaciones de los corticoides inhalados (CI) asociados a broncodilatadores de larga duración (BDLD) están bien definidas en las guías de práctica clínica. Sin embargo, existen áreas de incertidumbre acerca de su eficacia y seguridad. El objetivo de este documento es establecer un consenso de expertos acerca de estas áreas.
MétodoSe constituyó un grupo coordinador que realizó una revisión sistemática de la evidencia científica para proponer cuestiones que reflejaban áreas de incertidumbre relativas a la eficacia de los CI, los efectos adversos asociados a su empleo y los criterios para su retirada. Estas aseveraciones fueron sometidas a un panel de expertos mediante el método Delphi para comprobar el grado de consenso.
ResultadosParticiparon en el panel 25 expertos, que alcanzaron el consenso en la indicación de CI en el fenotipo mixto EPOC-asma, en su empleo en el paciente con agudizaciones frecuentes y en no añadir CI a BDLD para mejorar la función pulmonar del paciente con EPOC. En general, no hubo consenso en restringir el uso de CI motivado por sus efectos adversos. En cambio, el panel alcanzó el consenso en que la retirada del CI es factible pero debe hacerse de forma gradual y evaluando a corto plazo a los pacientes a los que se les retire.
ConclusionesExiste consenso en la indicación de CI en pacientes con fenotipo mixto EPOC-asma y agudizador frecuente. Se deben considerar los posibles efectos adversos, pero no existe consenso en sí justifican restringir su indicación. También existe consenso en que la retirada de CI es factible.
Chronic obstructive pulmonary disease (COPD) is highly prevalent. Data from Spain, based on the EPISCAN study,1 suggest that it affects 10.2% of Spanish adults aged between 40 and 80 years. Worldwide, it is the third cause of death2 and the fifth most burdensome disease in terms of disability-adjusted life years.3
Spanish COPD guidelines (GesEPOC) recommend the use of long-acting bronchodilators (LABD) as first line treatment, alone or in combination with other drug families (long-acting beta-agonists [LABA] and long-acting muscarinic antagonists [LAMA]), reserving the use of LABA+inhaled corticosteroids (IC) for frequent exacerbators with FEV1 <60% or mixed COPD-asthma phenotype, irrespective of severity.4 This is in line with the GOLD 2011 strategy that positions IC+LABA as treatment for patients with severe COPD with frequent exacerbations.5
Numerous Spanish and international observational studies performed in different care settings have detected intensive use of ICs alone or combined with LABD in COPD patients. In Spain, several studies6–8 have shown that over 60% of patients with mild COPD receive ICs (alone or in combination with LABDs), generally at high doses, irrespective of the care setting. This is in stark contrast with studies indicating that the mixed COPD-asthma phenotype is identified in less than 20% of patients,9 and that only one third of patients are frequent exacerbators.10
In some cases, COPD patients receive combination IC+LABD without a clear indication. Others return to clinical stability after an initially correct indication, but continue with the treatment in the long-term. The beneficial effect of IC in stable COPD in terms of reducing exacerbations and improving other health outcomes is widely supported in the literature. However, it remains unclear whether the response is universal or if it only occurs in a subgroup with certain characteristics, known as IC responders. Moreover, these benefits are accompanied by potentially serious side effects derived from long-term use. In view of these considerations, a consensus document has been developed with the aim of determining the level of agreement in various areas where the use of IC+LABD may be unclear, in order to establish criteria for the appropriate use of IC in stable COPD.
Material and MethodsThis consensus document was drawn up by a group of professionals involved in the treatment and research of COPD with the aim of standardizing the use of IC in stable COPD. Members of the consensus panel were selected on the basis of their research activities in COPD, their active participation in scientific forums, and their involvement in scientific societies dedicated to COPD care. The consensus process took place in 2 phases: in the first phase, a coordinator group was set up, consisting of the 6 authors of the document. The scientific evidence for the use of ICs in stable COPD was systematically reviewed. Three areas of interest were defined: (1) clinical benefits of the use of IC+LABD in stable COPD; (2) associated risks; and (3) evidence for IC withdrawal in stable COPD. In a second phase, after review of the evidence, areas of uncertainty in which expert opinion might converge in a consensus were detected for each area of interest. A total of 20 statements addressing these areas of uncertainty were drawn up and submitted to the panel for consensus. These questions were formulated exclusively by the members of the coordinator group, without the help of any external advisors, and submitted to the working group along with the complete review of the scientific evidence. Comments and suggestions for amending certain areas of the document or re-wording the questions for better understanding were noted. The expert group was given sufficient time to analyze the information provided and to make suggestions to the coordinator group before the voting process commenced.
The Delphi method was used to determine the level of agreement of the experts with regard to the statements on the use of IC in stable COPD. In this method, a questionnaire completed by a panel of experts is used to explore a route map for change. A summary of the expert opinions (in the form of quantitative evaluations and written comments) is provided as feedback to the same experts as part of the next round of the questionnaire.11 This methodology has already been used on other occasions in the area of COPD.12,13
The consensus participants individually assessed their level of agreement with each statement on a 9-point Likert scale, ranging from 1 (completely disagree with the statement) to 9 (completely agree with the statement). These scores were divided into 3 final groups: agree (7–9 points), neither agree nor disagree (4–6 points) and disagree (1–3 points). Voting took place in the first round in a face-to-face meeting using an interactive voting system (www.powervote.com). Any questions that did not achieve consensus in the first round of voting were submitted to a second, final round and drafted, compiled and analyzed using online survey software (www.surveymonkey.com). Consensus for each statement was established if at least 90% of the panel indicated their agreement with the statement in any of the 2 rounds, and the criterion for majority vote was met if at least 70% of the participants indicated agreement with the statement in any of the 2 voting rounds.
To analyze the data, the coordinating group collected all responses, transferred them to a Microsoft Office Excel spreadsheet (2010), and calculated the voting percentages for each block, as established above.
ResultsTwenty-seven physicians were invited to take part in the voting rounds (including pulmonologists, internal medicine and primary care specialists). Of these, 25 (92.6%) finally submitted their level of agreement with the statements.
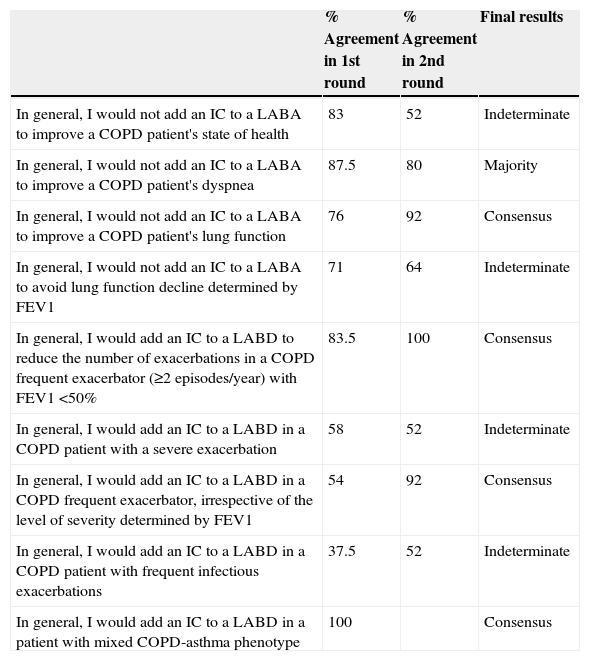
Clinical Efficacy of Inhaled Corticosteroids Combined With Long-Acting BronchodilatorsThe results of the 2 rounds of voting on this area of interest are presented in Table 1. In the first round of questions, consensus was reached on the use of ICs combined with LABD in the treatment of mixed COPD-asthma phenotype, with 100% agreement. In a second round of voting, consensus was obtained for 3 more statements referring to (1) the unsuitability of IC combined with LABDs to improve lung function in COPD patients (92% agreement); (2) combining ICs with LABDs for reducing exacerbations in patients with severe COPD (FEV1 <50%) and frequent exacerbations (100% agreement); and (3) the use of combination IC+LABD in frequent exacerbators, irrespective of the level of severity determined by FEV1 (92% agreement). Most participants in the working group agreed that IC should not be combined with LABD for improving dyspnea (80% agreement).
Statements on the Efficacy of ICs in Stable COPD, With the Percentage of Agreement in the First and Second Rounds, and the Consensus Result.
| % Agreement in 1st round | % Agreement in 2nd round | Final results | |
|---|---|---|---|
| In general, I would not add an IC to a LABA to improve a COPD patient's state of health | 83 | 52 | Indeterminate |
| In general, I would not add an IC to a LABA to improve a COPD patient's dyspnea | 87.5 | 80 | Majority |
| In general, I would not add an IC to a LABA to improve a COPD patient's lung function | 76 | 92 | Consensus |
| In general, I would not add an IC to a LABA to avoid lung function decline determined by FEV1 | 71 | 64 | Indeterminate |
| In general, I would add an IC to a LABD to reduce the number of exacerbations in a COPD frequent exacerbator (≥2 episodes/year) with FEV1 <50% | 83.5 | 100 | Consensus |
| In general, I would add an IC to a LABD in a COPD patient with a severe exacerbation | 58 | 52 | Indeterminate |
| In general, I would add an IC to a LABD in a COPD frequent exacerbator, irrespective of the level of severity determined by FEV1 | 54 | 92 | Consensus |
| In general, I would add an IC to a LABD in a COPD patient with frequent infectious exacerbations | 37.5 | 52 | Indeterminate |
| In general, I would add an IC to a LABD in a patient with mixed COPD-asthma phenotype | 100 | Consensus |
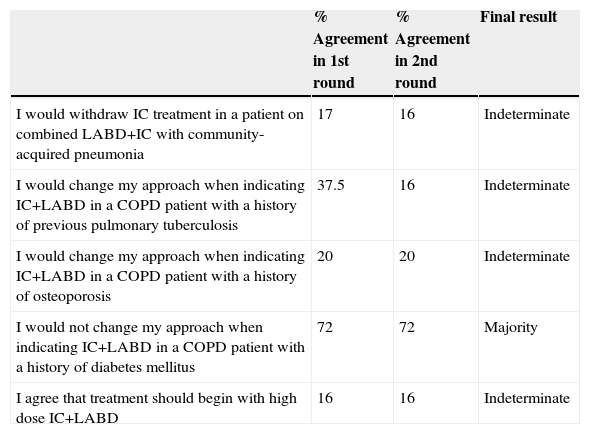
None of the 5 proposals on adverse effects reached consensus among the participants (Table 2), although the statement on not modifying treatment when prescribing combined ICs+LABD in patients with a history of diabetes achieved a majority vote (72% agreement).
Statements on the Side Effects of ICs in Stable COPD, With the Percentage of Agreement in the First and Second Rounds, and the Consensus Result.
| % Agreement in 1st round | % Agreement in 2nd round | Final result | |
|---|---|---|---|
| I would withdraw IC treatment in a patient on combined LABD+IC with community-acquired pneumonia | 17 | 16 | Indeterminate |
| I would change my approach when indicating IC+LABD in a COPD patient with a history of previous pulmonary tuberculosis | 37.5 | 16 | Indeterminate |
| I would change my approach when indicating IC+LABD in a COPD patient with a history of osteoporosis | 20 | 20 | Indeterminate |
| I would not change my approach when indicating IC+LABD in a COPD patient with a history of diabetes mellitus | 72 | 72 | Majority |
| I agree that treatment should begin with high dose IC+LABD | 16 | 16 | Indeterminate |
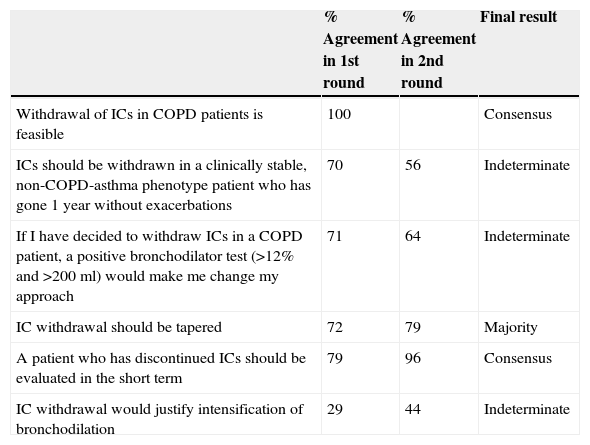
Six questions on the withdrawal of IC in stable COPD (Table 3) were submitted for consensus. Consensus was reached on the feasibility of IC withdrawal (100% agreement) in the first round of voting. In the second round, the statement regarding short-term follow-up after IC withdrawal reached consensus (96% agreement), and the majority (79%) of participants felt that IC withdrawal should be tapered.
Statements on the Withdrawal of ICs in Stable COPD, With the Percentage of Agreement in the First and Second Rounds, and the Consensus Result.
| % Agreement in 1st round | % Agreement in 2nd round | Final result | |
|---|---|---|---|
| Withdrawal of ICs in COPD patients is feasible | 100 | Consensus | |
| ICs should be withdrawn in a clinically stable, non-COPD-asthma phenotype patient who has gone 1 year without exacerbations | 70 | 56 | Indeterminate |
| If I have decided to withdraw ICs in a COPD patient, a positive bronchodilator test (>12% and >200ml) would make me change my approach | 71 | 64 | Indeterminate |
| IC withdrawal should be tapered | 72 | 79 | Majority |
| A patient who has discontinued ICs should be evaluated in the short term | 79 | 96 | Consensus |
| IC withdrawal would justify intensification of bronchodilation | 29 | 44 | Indeterminate |
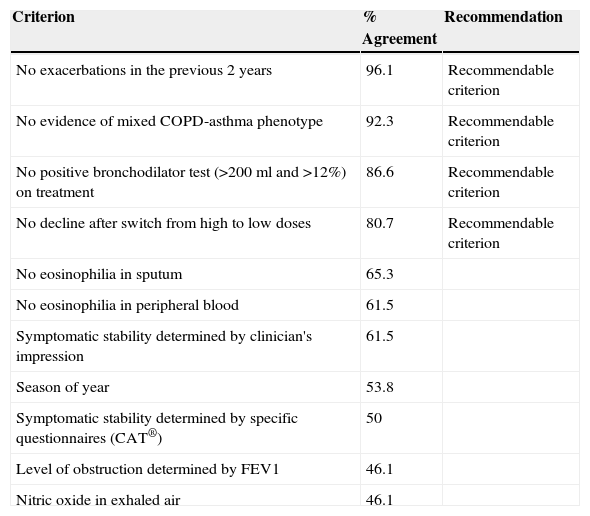
Regarding the criteria for indicating withdrawal of IC in stable COPD, consensus was obtained for patients with no exacerbations in the previous 2 years and in the absence of criteria for mixed COPD-asthma phenotype. There was less agreement on withdrawal if the patient did not present a positive bronchodilator test (>200ml and >12% FEV1 improvement) at the time of the evaluation and in the absence of decline after switching from high to intermediate IC doses. The general conclusion from these results is that IC withdrawal is possible if there is no evidence of mixed COPD-asthma phenotype, no exacerbations in the previous 2 years, and, with a lower level of agreement, in the absence of a positive bronchodilator test and decline after switching from high to intermediate IC doses (Table 4).
Criteria for IC Withdrawal in Stable COPD, With Level of Agreement and Final Recommendation of the Expert Group.
| Criterion | % Agreement | Recommendation |
|---|---|---|
| No exacerbations in the previous 2 years | 96.1 | Recommendable criterion |
| No evidence of mixed COPD-asthma phenotype | 92.3 | Recommendable criterion |
| No positive bronchodilator test (>200ml and >12%) on treatment | 86.6 | Recommendable criterion |
| No decline after switch from high to low doses | 80.7 | Recommendable criterion |
| No eosinophilia in sputum | 65.3 | |
| No eosinophilia in peripheral blood | 61.5 | |
| Symptomatic stability determined by clinician's impression | 61.5 | |
| Season of year | 53.8 | |
| Symptomatic stability determined by specific questionnaires (CAT®) | 50 | |
| Level of obstruction determined by FEV1 | 46.1 | |
| Nitric oxide in exhaled air | 46.1 |
The results of the process identify some areas of uncertainty in which it is difficult to achieve consensus. However, the expert panel is in overall agreement about certain statements.
The working group achieved consensus on the statement that all mixed COPD-asthma phenotype patients should continue to receive IC+LABA, irrespective of their disease severity or number of exacerbations. Although these patients are usually excluded from clinical trials with ICs in COPD, these data are in line with recent reviews showing that patients with a positive bronchodilator test have more chance of reducing exacerbations with the use of ICs,14 and that the response to ICs in COPD is associated with asthma-like characteristics, such as eosinophilic inflammation or raised levels of nitric oxide in exhaled air (FeNO).15,16
The statement that an IC should not be added to a LABA to improve lung function determined by FEV1 in a COPD patient achieved consensus. This opinion is in line with several recent systematic reviews17,18 showing that, while the use of ICs combined with LABAs improves FEV1, especially in the short term, long-term improvement is more modest (between 5 and 20ml) and of limited clinical significance.
For statements addressing the use of IC+LABA for reducing exacerbations in frequent exacerbators, the expert group agreed that in both situations (in patients with FEV1 <50% and irrespective of bronchial obstruction severity), ICs should be used along with LABAs. As might be expected, this is in line with the usual indication of IC+LABA in this situation (frequent exacerbations and severe airflow obstruction) in COPD clinical practice guidelines.4,5,19 It is also consistent with more recent studies showing that this combination can reduce exacerbations in patients with frequent exacerbations and FEV1 <70%. These data suggest, then, that the effect of ICs is probably not related with the level of severity, but rather with an exacerbator phenotype, particularly if characteristics consistent with the COPD-asthma phenotype are observed.20–22
Questions on the side effects of long-term use of ICs did not achieve consensus, and answers differed widely among the group of experts. This shows, on the one hand, the lack of evidence on the side effects of ICs, and on the other, a lack of understanding of the real impact of these side effects on the COPD population usually seen in the clinical setting.
The expert group reached consensus on the statement that withdrawal of ICs in COPD is feasible. Although the evidence on the withdrawal of ICs is in general limited and very heterogeneous, recent studies support the idea proposed by GesEPOC that IC withdrawal is safe in stable patients with no exacerbations.23,24 The best strategy for IC withdrawal has yet to be defined. However, it seems logical that, since exacerbations associated with IC withdrawal tend to occur in the first months after discontinuation, COPD patients in this situation should be followed-up as soon as possible. The expert group agreed that patients discontinuing ICs should be seen in the outpatient clinic shortly after withdrawal. New evidence has shown that tapered withdrawal of ICs in even the most severely affected COPD patient is safe and will not lead to exacerbations in the long-term. However, more studies are needed to characterize patients in whom withdrawal is safe.25
Finally, the expert group reached consensus on 2 clinical requirements for IC withdrawal in COPD patient (absence of mixed COPD-asthma phenotype and absence of exacerbations in the previous 2 years). These were the most highly recommended, and a further 2 requirements received a majority vote (absence of positive bronchodilator test and absence of decline after switching from high to intermediate dose ICs). These criteria are intended as general guidelines to help clinicians in the management of COPD patients, but they must be validated in prospective studies or randomized clinical trials.
The document has its limitations, including the fact that, inherent to the nature of this type of document, results can only be taken as expert opinions. The recommendations need to be backed up by clinical studies to clarify the areas of uncertainty detected.
To conclude, this document has highlighted areas of consensus among experts on the use of ICs in the mixed COPD-asthma phenotype and in frequent exacerbators, on the unsuitability of ICs for improving lung function, and on the feasibility of IC withdrawal. Nevertheless, uncertainty regarding other aspects of this therapy could explain why the use of these drugs differs so widely among clinicians. If clinical practice is to improve, studies must be performed to examine and clarify these aspects.
Conflict of InterestsBernardino Alcázar Navarrete has received fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Grupo Ferrer, GSK, Laboratorios Menarini, Novartis, Pfizer, Takeda for speaking engagements and/or scientific advice.
Ciro Casanova has received fees from Almirall, AstraZeneca, GlaxoSmithKline, Novartis for scientific advice and/or speaking engagements.
Marc Miravitlles has received fees from Almirall, AstraZeneca, Boehringer Ingelheim, Grupo Ferrer, GlaxoSmithKline, Grifols, Laboratorios Esteve, Pfizer, Novartis, Gebro Pharma and Takeda for scientific advice and/or speaking engagements.
Pilar de Lucas has received fees from Almirall, Boehringer Ingelheim, Novartis, Teva, Takeda for scientific advice and/or speaking engagements.
Juan Antonio Riesco has received fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Grupo Ferrer, GSK, Laboratorios Esteve, Laboratorios Menarini, Novartis, Pfizer, Takeda for giving speaking engagements and/or scientific advice.
José Miguel Rodríguez González-Moro has received fees from Almirall, Boehringer Ingelheim, Chiesi, GSK, Laboratorios Menarini, Novartis, Pfizer for speaking engagements and/or scientific advice.
This consensus study was made possible by an unrestricted grant from Novartis. The sponsor did not participate in any aspect of its design, conduct or drafting, their input being limited to the logistical organization. Participants did not receive any financial compensation for participation. The opinions expressed in this study are solely and exclusively those of the authors and reflect those of the consensus participants.
A. Fernández Villar (Complejo Hospitalario Universitario de Vigo, Pontevedra). A. Ruiz Sancho (Hospital de Alta Resolución de Loja, Granada). A. Huerta (Hospital Clínic, Barcelona). B. García Cosío (Hospital Universitario Son Espases, Palma de Mallorca). C. Esteban (Hospital Galdakao-Usasolo, Vizcaya). F. Ortega Ruiz (Hospitales Universitarios Virgen del Rocío, Seville). G. Peces Barba (Fundación Jiménez Díaz, Madrid). J.A. Quintano (Centro de Salud Lucena I, Cordoba). J.L. López Campos (Hospitales Universitarios Virgen del Rocío, Seville). J.L. Viejo Bañuelos (Burgos). J.J. Soler Cataluña (Hospital Universitario Arnau de Villanova, Valencia). J.P. de Torres (Clínica Universitaria de Navarra, Navarre). M. Román Rodríguez (Centro de Salud Son Pisa, Palma de Mallorca). M. Calle Rubio (Hospital Clínico San Carlos, Madrid). P. García Sidro (Hospital de la Plana, Castellon). P. Sobradillo Ecenarro (Hospital Universitario Txagorritxu, Alava). P. J. Marcos (Complejo Hospitalario Universitario A Coruña, Corruna). P. Almagro Mena (Hospital Mutua de Terrasa, Barcelona). R. Agüero Balbín (Hospital Universitario Marqués de Valdecilla, Santander). R. Malo de Molina (Hospital Puerta de Hierro, Madrid).
The members of the Working Group “Consensus document on the appropriate use of inhaled corticosteroids in COPD” listed in Appendix A.
Please cite this article as: Alcázar Navarrete B, Casanova C, Miravitlles M, de Lucas P, Riesco JA, Rodríguez González-Moro JM. Documento de consenso “Uso adecuado de los corticoides inhalados en la enfermedad pulmonar obstructiva crónica”. Arch Bronconeumol. 2015;51:193-198.