There are three major challenges in the diagnosis of malignant pleural mesothelioma: mesothelioma must be distinguished from benign mesothelial hyperplasia; malignant mesothelioma (and its subtypes) must be distinguished from metastatic carcinoma; and invasion of structures adjacent to the pleura must be demonstrated. The basis for clarifying the first two aspects is determination of a panel of monoclonal antibodies with appropriate immunohistochemical evaluation performed by highly qualified experts. Clarification of the third aspect requires sufficiently abundant, deep biopsy material, for which thoracoscopy is the technique of choice. Video-assisted needle biopsy with real-time imaging can be of great assistance when there is diffuse nodal thickening and scant or absent effusion. Given the difficulties of reaching an early diagnosis, cure is not generally achieved with radical surgery (pleuropneumonectomy), so liberation of the tumor mass with pleurectomy/decortication combined with chemo- or radiation therapy (multimodal treatment) has been gaining followers in recent years. In cases in which surgery is not feasible, chemotherapy (a combination of pemetrexed and platinum-derived compounds, in most cases) with pleurodesis or a tunneled pleural drainage catheter, if control of pleural effusion is required, can be considered. Radiation therapy is reserved for treatment of pain associated with infiltration of the chest wall or any other neighboring structure. In any case, comprehensive support treatment for pain control in specialist units is essential: this acquires particular significance in this type of malignancy.
El diagnóstico del mesotelioma pleural maligno presenta 3 importantes retos: es necesario distinguir entre hiperplasia mesotelial benigna y mesotelioma, entre mesotelioma maligno (con subtipos) y carcinoma metastásico, y también se requiere demostrar la invasión de estructuras vecinas a la pleura. Para aclarar los 2 primeros aspectos hay que basarse en un panel de anticuerpos monoclonales con adecuado estudio inmunohistoquímico–realizado por manos muy expertas–y para el tercero hay que apoyarse en biopsias suficientemente amplias y profundas, y la toracoscopia es la técnica de elección. La biopsia con aguja guiada con técnicas de imagen en tiempo real puede ser de gran ayuda cuando existe marcado engrosamiento nodular difuso y derrame pequeño o ausente. Dadas las dificultades de un diagnóstico precoz, es infrecuente que se consiga un tratamiento curativo mediante cirugía radical (pleuroneumonectomía), por lo que en los últimos años está ganando adeptos la liberación de masa tumoral mediante pleurectomía/decorticación, con asociación de quimio y radioterapia a las técnicas quirúrgicas (terapia multimodal). En los casos en que la cirugía no es factible se plantea la quimioterapia (combinando pemetrexed y compuestos de platino en la mayoría de los casos), con pleurodesis o colocación de un catéter pleural tunelizado si se requiere el control del derrame pleural, y se reserva la radioterapia para el tratamiento del dolor asociado a infiltración de la pared torácica o cualquier otra estructura vecina. En todo caso, es esencial un completo tratamiento de soporte para el control del dolor (que adquiere particular protagonismo en esta neoplasia) en unidades especializadas.
Mesothelioma is a tumor derived from the mesothelium, the mesoderm cell layer that lines the embryonic coelom (body cavity). This tissue subsequently develops into the pleura, pericardium, peritoneum and the testicular tunica vaginalis. Because of its mesodermal origin, the mesothelium can potentially develop an epitheloid component and a sarcomatous component. Since the 1950s, mesothelioma has been associated with asbestos,1 particularly blue asbestos (crocidolite) and white asbestos (cristolite). It has also been associated with erionite, a natural soil contaminant that occurs in several regions throughout the world, but especially in Cappadocia (Turkey), where a very high incidence of mesothelioma has been recorded, although this may also be due to a certain genetic susceptibility.2 Approximately 80% of cases of mesothelioma show a cause-effect relationship with workplace exposure to asbestos in a wide spectrum of professions,3 but the possibility of environmental exposure must also be considered. This generally originates from local mines or factories handling this mineral, or contamination from the clothes of asbestos workers.4 A dose–response relationship between accumulated asbestos exposure (high levels of exposure, duration of exposure or both) and malignant mesothelioma has been shown, and there is no threshold below which the risk of contracting the disease can be ruled out.5–7 Mesothelioma can develop in any of the above-mentioned mesodermal structures, but the most common presentation (over 90% of cases) is pleural. Nevertheless, the incidence of the disease is relatively low, ranging from 7 cases per million inhabitants/year in Japan to 40 per million/year in Australia, mainly in line with asbestos exposure rates in previous decades.8 In Europe, incidence is approximately 20 cases per million/year, but this rate varies widely between countries, and is also related to asbestos exposure in the past. The latency period between exposure and manifestation of the disease is long–generally around 40 years, while outlying values (up to 75 years in the series of Bianchi et al.) vary greatly9–suggesting that the worldwide incidence may still be expected to rise. Based on asbestos exposure figures, the incidence of mesothelioma in Europe is expected to peak in around 2020, and will vary widely between countries.10
For this review article, a PubMed search (http://www.ncbi.nlm.nih.gov/pubmed), updated 18 March 2014, was performed, combining the terms malignant + pleural + mesothelioma. A total of 4670 articles, including 736 reviews, were found. The most relevant articles selected as the basis for this review of malignant pleural mesothelioma were detailed prospective studies in large series, evidence-based clinical guidelines and some papers on very specific areas, such as biomarkers or innovative techniques with outlooks on the future.
Diagnosis of Malignant Pleural MesotheliomaThe initial clinical presentation of mesothelioma may be dyspnea, generally associated with developing pleural effusion. Pleural pain, not clearly related with breath movements, is also a common manifestation. Weight loss and other symptoms are rare in the early stages, but as the condition advances, marked hemithorax retraction is often observed and pain becomes particularly intense and persistent.
Imaging Techniques in the Diagnosis of MesotheliomaAlthough chest X-ray is the first step in diagnosis, and can provide information on the presence of effusion, diffuse pleural thickening or masses, computed tomography (CT), preferably with contrast medium, is essential for the proper evaluation of the patient and for determining the diagnostic procedure: diffuse pleural thickening with prominent lymph nodes suggests mesothelioma, particularly in patients with a history of exposure to asbestos of any type.11 However, CT has poor sensitivity for the evaluation of mediastinal node involvement or contralateral or peritoneal lung pleural involvement. Positron emission tomography (PET) is much more useful for studying these regions and the possibility of distant metastases, particularly if it is combined with CT (PET-CT).12 PET-CT is particularly useful for pre-surgery staging of malignant pleural mesothelioma, for evaluating treatment response, and for detecting possible relapse.13 However, its sensitivity and specificity for detecting N2 disease in mesothelioma is low,14 and false negatives may be found in tuberculous pleurisy,15 empyema16 or in patients with a history of previous pleurodesis.17
Magnetic resonance imaging (MRI) provides greater contrast than CT for defining tumor chest wall invasion, but it cannot reliably detect metastatic disease.18
Pleural Fluid StudiesThoracocentesis can provide data suggestive of mesothelioma, but rarely diagnostic: high levels of hyaluronic acid (>100000ng/ml) are highly indicative of malignant pleural mesothelioma.19 Elevated hyaluronic acid also has good prognostic values, with higher levels being associated with better survival.20
Adenosine deaminase (ADA) levels may be raised in mesothelioma patients,21 but before they are labeled as ADA false positives, it should be remembered that malignant mesothelioma may sometimes coexist with tuberculous pleurisy, so a culture for Mycobacterium tuberculosis is recommended in these cases.22
Pleural fluid cytology may reveal mesothelioma, but it is often difficult to distinguish between benign and malignant mesothelial hyperplasia.23 This test is also unable to determine the invasive character of the tumor, currently considered an essential feature for a definitive diagnosis).24 However, in some exceptional cases, cytology may be combined with imaging techniques for the evaluation of extrapleural invasion.25 Immunocytochemical and immunohistochemical techniques are also essential for differentiating between mesothelioma and metastatic adenocarcinoma in the pleura,26 and require biopsy tissue or paraffin-embedded preparation of the cell button after centrifugation of a sufficient volume of pleural fluid (>100ml). A diagnosis of mesothelioma can be reliably made when all the following conditions are found: atypical mesothelial proliferation in the pleural fluid + cell block immunohistochemistry consistent with mesothelioma + diffuse pleural thickening with nodularity + absence of any intra- or extrapulmonary mass suggestive of another primary tumor.27 However, a diagnosis of malignant mesothelioma generally has legal implications. Therefore, when surgery is proposed an attempt should always be made to obtain sufficient tissue specimens for more accurate tumor typing.28
Histological Diagnosis of Malignant Pleural MesotheliomaThe classic types of malignant pleural mesothelioma are epithelioid, sarcomatous and biphasic, but there are also rare subtypes, such as desmoplastic mesothelioma (that may be confused with benign fibrous pleuritic), small cell mesothelioma and lymphohistiocytoid mesothelioma (that may be confused with lymphoma). Immunohistochemical evaluation is essential for identifying these types. However, no single marker has 100% sensitivity and specificity for mesothelioma, so various monoclonal antibody panels must be determined, of which at least 2 must be positive for mesothelioma. In the epithelioid subtype, these should be preferably calretinin (particularly useful if the nucleus, in addition to the cytoplasma, is stained), Wilms’ tumor antigen 1 (WT-1) or epithelial membrane antigen (EMA) or wide-spectrum, low molecular weight cytokeratins, such as CK5 OR CK6, and 2 negative markers, such as Ber-EP4 (membrane marker) and thyroid transcription factor 1 (TTF-1, nuclear marker). Carcinoembryonic antigen (CEA) is very useful for distinguishing metastatic carcinoma, particularly of pulmonary origin, from mesothelioma (in which it is practically always negative), and if mesothelioma is suspected in a woman, endoplasmic reticulum (ER) expression should also be investigated: this never occurs in mesothelioma but is a common feature of metastatic breast tumors28 (see Table 1). When the tumor contains a sarcomatous component, it often needs to be distinguished from metastases, such as squamous cell lung cancer or transitional cell carcinoma. Although some antibodies used for epithelioid mesothelioma are equally valid for sarcomatous types, some others are often needed for firm evidence, such as p63 and MOC 31 (see Table 2).
Immunohistochemical Markers for Differentiating Epithelioid Mesothelioma From Other Metastatic Pleural Tumors.
| Antibody | Diagnostic value | Epithelioid mesothelioma | Adenocarcinoma |
|---|---|---|---|
| Calretinin | Essential | +++ (nucleus and cytoplasm) | ± (cytoplasm) |
| WT-1 | Useful | ++ (nuclear) | (lung) |
| EMA | Useful | ++ (membrane) | +++ (cytoplasm) |
| CK5/CK6 keratins | Useful | ++ (cytoplasm) | – |
| Monoclonal CEA | Very useful | – | ++ (cytoplasm) |
| Ber-EP4 | Very useful | ± (membrane) | +++ (membrane) |
| TTF-1 | Very useful | – | ++ (nucleus, lung) |
| B72.3 | Very useful | – | +++ (cytoplasm, lung) |
| ER | Very useful | – | ++ (nucleus, breast) |
CEA: carcinoembryonic antigen; EMA: epithelial membrane antigen; ER: endoplasmic reticulum marker; TTF-1: thyroid transcription factor-1; WT-1: Wilms tumor antigen 1.
Source: Adapted from Scherpereel et al.28
Immunohistochemical Markers for Differentiating Sarcomatous Mesothelioma From Squamous Cell or Transitional Cell Carcinoma.
| Antibody | Diagnostic value | Sarcomatous mesothelioma | Squamous cell or transitional cell carcinoma |
|---|---|---|---|
| Calretinin | Useful | +++ (nucleus and cytoplasm) | + (cytoplasm) |
| WT-1 | Useful | ++ (nucleus) | |
| CK5/CK6 keratins | Not useful | ++ (cytoplasm) | +++ (cytoplasm) |
| p63 | Very useful | – | +++ (nucleus) |
| Ber-EP4 | Very useful | ± (membrane) | +++ (cytoplasm) |
| MOC 31 | Useful | ± (membrane) | +++ (membrane) |
p63: p53 homolog, but more useful for differential diagnosis; WT-1: Wilms tumor antigen 1.
Source: Adapted from Scherpereel et al.28
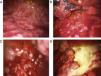
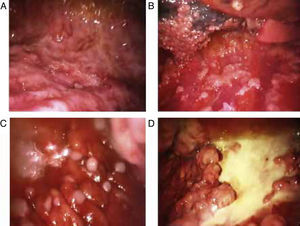
Blind pleural needle biospy (without real-time imaging techniques) yields unsatisfactory results in the diagnosis of mesothelioma, not only due to lack of control when selecting the exact sampling point, but also due to the small size of such samples. In case of diffuse nodular pleural thickening, needle biopsy yield may improve considerably if it is performed with the aid of CT29,30 or real-time ultrasound.31 In 1 study, a diagnostic yield of 75% was achieved in cases in which biopsies were >10mm, falling to only 8% if specimens were smaller.32 This finding clearly supports the use of thoracoscopy in the diagnosis of malignant pleural mesothelioma. Thoracoscopy (or pleuroscopy) can be performed with local anesthesia and intravenous analgesia/sedation.33 In our hospital, we have used this technique to diagnose more than 80 pleural mesotheliomas34 (see Fig. 1). Video-assisted thoracoscopic surgery (VATS) improves tumor staging, particularly in the mediastinal region, and can be used for pleurectomy/decortication, although more resources, including general anesthesia and tracheal intubation, will be required.35 The yield from pleuroscopy, also known as “medical thoracoscopy”, is suboptimal in mesothelioma with sarcomatous component,36 and for this subtype, more representative samples can be obtained with VATS or mini-thoracotomy.37
Various thoracoscopic images of malignant pleural mesothelioma. (A) Massive epithelioid malignant mesothelioma invasion of the parietal pleural. (B) Diffuse epithelioid malignant mesothelioma infiltration of parietal and visceral pleura. (C) “String of pearls” nodes in massive epithelioid invasion of the parietal pleural. (D) Pleural plaques and sarcomatous mesothelioma masses in the parietal pleura.
Despite a clearly defined risk population (individuals exposed to some form of asbestos), one of the major challenges we face is the lack of tools for making a sufficiently early diagnosis to allow radical treatment of the disease. This can only be achieved with biomarkers that can detect disease before the development of effusion or diffuse pleural thickening, along with sufficiently sensitive and specific imaging techniques.
Biomarkers in the Diagnosis of Malignant MesotheliomaThe biomarker generating the most interest in recent years is soluble mesothelin. This protein is closely correlated with tumor size and progression in epithelioid mesothelioma (although it tends to be negative in sarcomatous type).38 However, values are affected by renal function, and one of the major problems is the identification of the right cutoff point for distinguishing between benign and malignant pleural involvement.39 In any case, it seems that pleural fluid mesothelin levels are more useful than serum levels, and this greatly limits the value of this biomarker in the early diagnosis of subjects with a history of asbestos exposure but no pleural effusion. If screening suggests a low probability of mesothelioma, low mesothelin levels may help to rule out the disease, while high levels would justify the use of more invasive techniques in the case of suspected mesothelioma.40–42 In all events, mesothelin appears to be more useful for monitoring treatment than for the differential diagnosis of pleural effusion.43
Efforts to overcome the problems of mesothelin and other markers include a recent study of the ability of fibulin-3 to distinguish between healthy subjects with a history of asbestos exposure and mesothelioma patients, and even between mesothelioma and other malignant or benign diseases of the pleura. Thus, Pass et al. found that fibulin in plasma had 96.7% sensitivity and 95.5% specificity at a cutoff point of 52.8ng/ml. When patients with relatively early stage mesothelioma were compared with individuals exposed to asbestos but with no evidence of disease, sensitivity and sensitivity of a cutoff point of 46ng/ml of fibulin-3 were 100% and 94.1%, respectively.44 These excellent results still need proper external validation, and it must be remembered that sample extraction and processing techniques may significantly affect the results: in the case of fibulin, determination in plasma (before activation of the coagulation process) is much more reliable than in serum, due, as with other markers, to thrombin-fibulin interactions.45
In recent years, gene expression in mesothelioma has been the object of intensive research,46 and the expression of certain proteins has been targeted, including aquaporin-1, associated with selective water transport through the membrane and with cell proliferation.47 Of special interest is the work on micro-RNA (miRNA) in mesothelioma. These are short, non-protein coding RNAs (17–22 nucleotides) that regulate gene expression and play an important role in oncogenesis.48 They have high tissue specificity for detecting the origin of a tumor and also for distinguishing mesothelioma from other metastatic pleural tumors.49 Consequently, miRNA detection in peripheral blood may become an excellent marker for mesothelioma in the near future.50
Treatment of Malignant Pleural MesotheliomaPleural mesothelioma generally responds poorly to chemotherapy and radiotherapy and surgery is rarely curative, because the tumor is usually diagnosed too late. For this reason, it is essential to carefully evaluate the patient before selecting the best treatment modality. If radical treatment is being considered, lung and cardiac function, other comorbidity factors, and the physical and psychological status of the patient must be assessed. Choice of one or other of the various available options is dictated by the clinical situation and tumor extension (TNM) determined by imaging techniques. However, none of the currently available imaging techniques is precise enough to determine the “T” and “N” components in malignant pleural mesothelioma, and post-surgical staging often widely exceeds pre-surgical findings.51 Until more robust TNM staging can be defined, the system established by the
Union Internationale contre le Cancer (UICC) should be used52 (see Table 3).
Malignant Pleural Mesothelioma TNM Staging.
| Stage | Tumor extension |
|---|---|
| T1 | Unilateral parietal pleural involvement |
| T1a | Parietal pleural involvement with or without mediastinal pleural or diaphragmatic pleural involvement, but with no visceral pleural involvement. |
| T1b | Parietal pleural involvement with focal visceral pleural involvement. |
| T2 | Unilateral parietal or visceral involvement with invasion of underlying lung or diaphragmatic muscle |
| T3 | Unilateral involvement of any pleural area, with invasion of at least one of the following structures: endothoracic fascia, mediastinal fat, soft tissue of chest wall (focal) or nontransmural invasion of the pericardium |
| T4 | Involvement of any pleural zone with invasion of at least one of the following structures: internal surface of the pericardium (with or without effusion), peritoneum, mediastinal structures, contralateral pleura, spine, diffuse invasion of the chest wall (with or without rib destruction) |
| N0 | No lymph node involvement |
| N1 | Ipsilateral bronchopulmonary or hilar lymph node involvement |
| N2 | Ipsilateral mediastinal lymph node involvement, including the ipsilateral internal mammary and/or peridiaphragmatic nodes |
| N3 | Contralateral mediastinal, contralateral internal mammary and/or contralateral supraclavicular lymph node involvement |
| M0 | No extrathoracic metastases |
| M1 | Extrathoracic, hematogenous or non-regional lymph node metastases |
| Stage I | T1aN0 (1A); T1bN0 (1B) |
| Stage II | T2N0 |
| Stage III | Any T3, N1 or N2 |
| Stage IV | Any T4, N3 or M1 |
The main objective of surgery is the gross resection of all malignant growth. The assumption is that this leads to longer survival and that patients who have gross evidence of remaining tumor will have poorer survival.53 However, accumulated evidence suggests that full resection (macro- and microscopic) of mesothelioma is impossible, irrespective of the surgical technique used. Therefore, it is accepted that surgery is aimed at local disease control, elimination of pleural effusion, release of lung trapped by the tumor, improvement of ventilation/perfusion disorders and relief of pain from chest wall invasion.54 All these considerations are particularly applicable to epithelioid mesothelioma, since the sarcomatous or biphasic types have a worse prognosis, and as such, are poorer candidates for any type of surgery.51
Extrapleural PneumonectomyExtrapleural pneumonectomy involves resection of the lung and the parietal pleura, and is usually completed with ipsilateral resection of the pericardium and diaphragm, in addition to systematic dissection of the mediastinal lymphatic chains. Perioperative mortality is around 5% in highly experienced hospitals. However, morbidity is high and includes cardiac and respiratory complications, of particular relevance in the unipulmonary situation encountered after extrapleural pneumonectomy (EPP). Other complications include bronchopleural fistula, empyema and bleeding.55,56 In any event, this intervention commonly involves persistent residual gross and microscopic disease, so it should be planned within the context of multimodal therapy, based on the combined use of surgery, chemotherapy and radiation therapy.57 Hyperthermia, combined with chemotherapy, or local photodynamic therapy have occasionally been used.58 Many multimodal treatment protocols recommend chemotherapy as pre-surgery induction treatment (neoadjuvant chemotherapy), with radiation therapy administered to the affected hemothorax after resection.59 Nevertheless, the most recent guidelines recommend avoiding surgery if progressive disease is observed after neoadjuvant chemotherapy; besides, EEP is only recommended in the context of controlled clinical trials performed by specialized teams of investigators.27,28
Pleurectomy/DecorticationAlthough pleurectomy/decortication, aimed primarily at releasing the lung and the chest wall from constriction caused by the tumor, is associated with a higher risk of local relapse than EEP, it involves fewer complications.60 The best candidates for this type of surgery are patients with gross diffuse tumors in the parietal wall, but only focal sites in the viscera.54 The procedure can be performed using VATS, with the advantage of minimizing morbidity associated with thoracotomy61 and the possibility of performing pleurodesis during the same intervention, if resection cannot be completed.
EEP constitutes a more radical approach, but in recent years its advantages over pleurectomy/decortication have been questioned.62,63 No superiority was observed for either technique in a recent randomized study performed in the United Kingdom (the Mesothelioma and Radical Surgery [MARS] study).64 Nevertheless, the MARS study has been severely criticized, due to significant deviations from the original protocol design and the number of patients finally included in one of the study groups.65 In any case, although some groups with extensive experience in both techniques find it unacceptable to forgo resection of grossly visible tumor (giving a poorer prognosis),54 the idea of resecting the greatest tumor volume possible, while preserving the underlying lung and combining surgery with chemotherapy and radiation therapy in multimodal treatment, is gaining ground.66
Radiation Therapy in Malignant MesotheliomaRadical radiation therapy administered to the whole hemithorax is seriously limited by the risk of damaging critical organs, such as the lung, liver, heart, bone marrow and esophagus, although these negative effects are being palliated by optimized application techniques.67 However, there is no convincing evidence that this alone prolongs survival in mesothelioma.68 On the other hand, palliative radiation therapy plays an important role in the control of pain from chest wall infiltration.69,70 The classic recommendation has been to administer prophylactic radiation therapy to avoid tumor seeding in the thoracoscopy or thoracotomy scars,71,72 but this practice is not supported by available evidence, and is no longer advised.27,28
Chemotherapy, Immunotherapy and Other Tailored TreatmentsRecent clinical guidelines advise against delaying the administration of chemotherapy, which must be considered before the patient's functional status begins to deteriorate.27,28 The combination of various agents, including pemetrexed and platinum compounds, generally produces better results than monotherapy.73,74 Current trends in research are oriented toward new therapeutic targets focused on controlling angiogenesis and apoptotic pathways via specific ligands, such as platelet derived growth factor (PDGF, that is quite often expressed in the mesothelioma and associated with poorer survival) and mesothelin (expressed only in the epithelioid subtype), among others.75,76 Immunotherapy is another aspect of multimodal therapy that can be effective in the treatment of mesothelioma, since this tumor uses regulatory T-cells (Tregs) and M2 macrophages to escape the immune system. New therapeutic strategies combining cytoreductive surgery, chemotherapy, immunotherapy and radiation therapy may lead to better disease control.77 There is a wide spectrum of possible approaches for achieving notably synergic antitumor effects, from passive immunotherapy (using cytokines or specific antibodies) to immune response modulation using dendritic cells or others.78–80
PleurodesisControl of pleural effusion is a priority in most patients with malignant pleural mesothelioma, and a good option for this is talc pleurodesis. However, in our experience, pleurodesis tends to fail more often in this tumor than in others, possibly due to difficulties in achieving re-expansion of the lung that has been trapped by the tumor.81 Previous studies by our group show that the extension of the tumor in the pleural cavity has a negative effect on pleurodesis.82 It seems likely, then, that other biological factors, unidentified to date, are also involved. According to recent in vitro experiments carried out by our group, malignant mesothelial cells are more resistant to the action of talc than other cell lines, as observed in both the modulation/blocking of angiogenesis and in cell proliferation (unpublished data).
When pleurodesis fails, or is considered unfeasible due to massive lung entrapment by the tumor, the best option is to place a tunneled pleural catheter for drainage of pleural fluid at home. This procedure also induces spontaneous pleurodesis in a considerable number of cases.83–86
It is important to remember that previous pleurodesis does not rule out the need for surgical resection of mesothelioma, whether by EEP or pleurectomy/decortication.54
Future OutlookMolecular biology and nanotechnology are coming together in the newly emerging concept of “theranostics”, the aim of which is to combine diagnosis and treatment in the same procedure with the use of drugs that specifically target each malignancy subtype. If the right ligands were found, they could be used in PET or single photon emission computed tomography (SPECT) for the early diagnosis of mesothelioma.87–89 Highly sensitive probes combined with biofluorescent techniques that can detect tumors in animal models have been developed.90,91 Techniques based on labeled antibodies or nanoparticles with nuclear magnetic resonance are available,92,93 and the prospect of eventually using these in humans is good. Until such time, however, it seems more realistic to concentrate on the search for markers that are detectable in peripheral blood and sufficiently sensitive and specific for the diagnosis of malignant mesothelioma. Along with imaging techniques, the most promising field appears to be comprehensive screening for early mesothelioma markers using proteomics. This can simultaneously analyze the profiles of large numbers of proteins (over 1000), in panels configured to achieve the greatest diagnostic sensitivity and specificity.94,95 As explained above, miRNA detection in peripheral blood is another emerging field in the hunt for a sufficiently early diagnostic test for mesothelioma.
Gene therapy has often been proposed in mesothelioma to compensate for the poor results of immune therapy in locally advanced disease. To this end, different strategies, such as “suicide genes” (which make the tumor sensitive to certain drugs), administration of tumor suppressor genes, or the transfer of immunomodulatory genes to the pleural space have been proposed.96–99 Results in the clinic are rather disheartening to date due to vector-related problems and relative inefficiency in controlling large tumor masses. However, gene therapy is most likely to be incorporated into the multimodal strategy, and when combined with nanotechnology techniques it will contribute very significantly to the improvement of treatment of malignant pleural mesothelioma in the future.
FundingPart of the funding of the research performed by our group in this article, was funded by Project PI-0358-2010, of the Health Council of the Government of Andalusia.
Conflict of InterestsThe author states that he has no conflict of interests.
Please cite this article as: Rodríguez Panadero F. Diagnóstico y tratamiento del mesotelioma pleural maligno. Arch Bronconeumol. 2015;51:177–184.