Bronchiectasis is one of the most common comorbidities in severe asthma. However, the mechanisms by which asthma promotes the development and progress of this condition are not well defined. This study aimed to analyze the inflammatory phenotypes and quantify the expression of proinflammatory and remodeling cytokines in asthma patients with and without bronchiectasis.
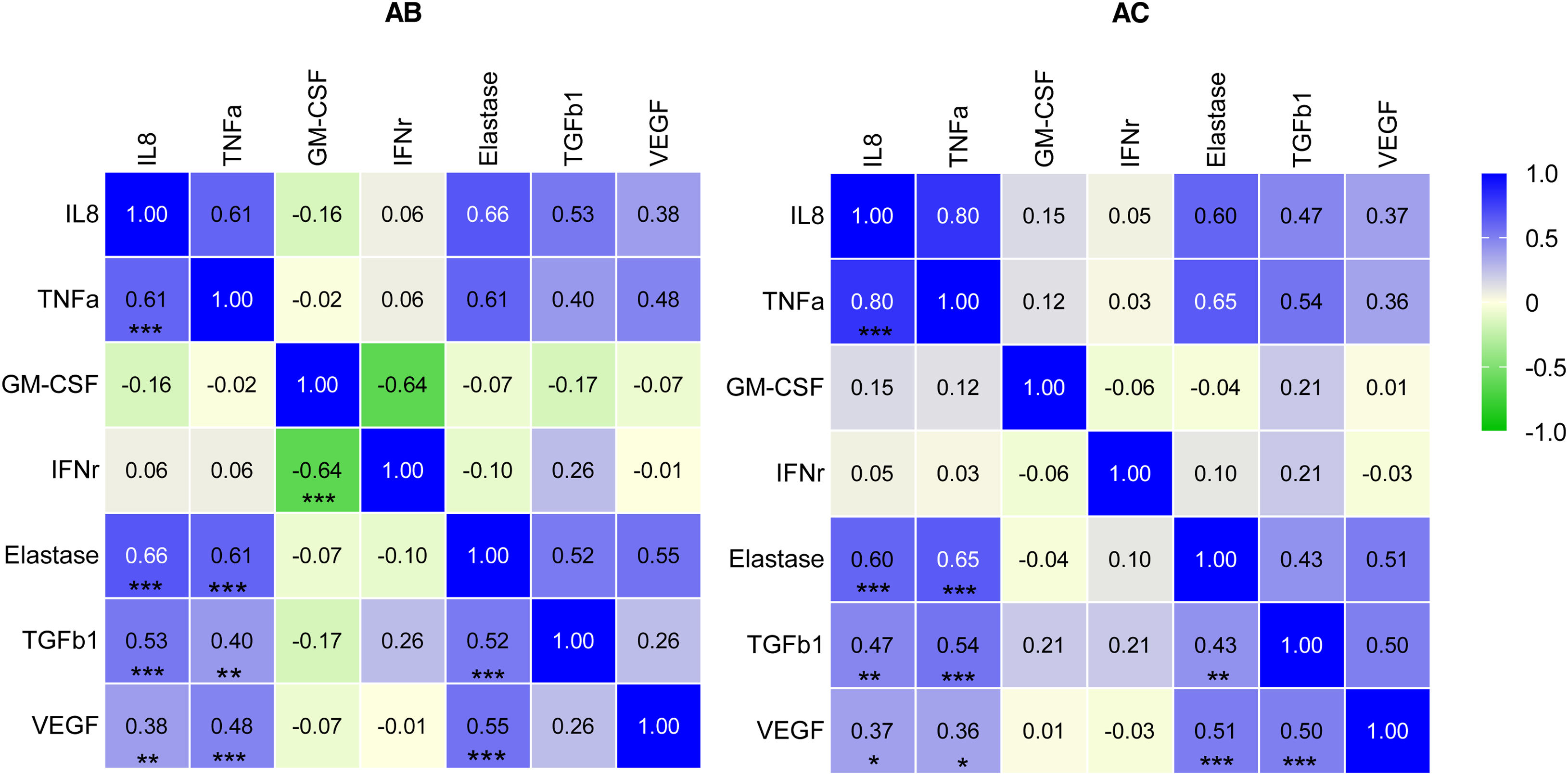
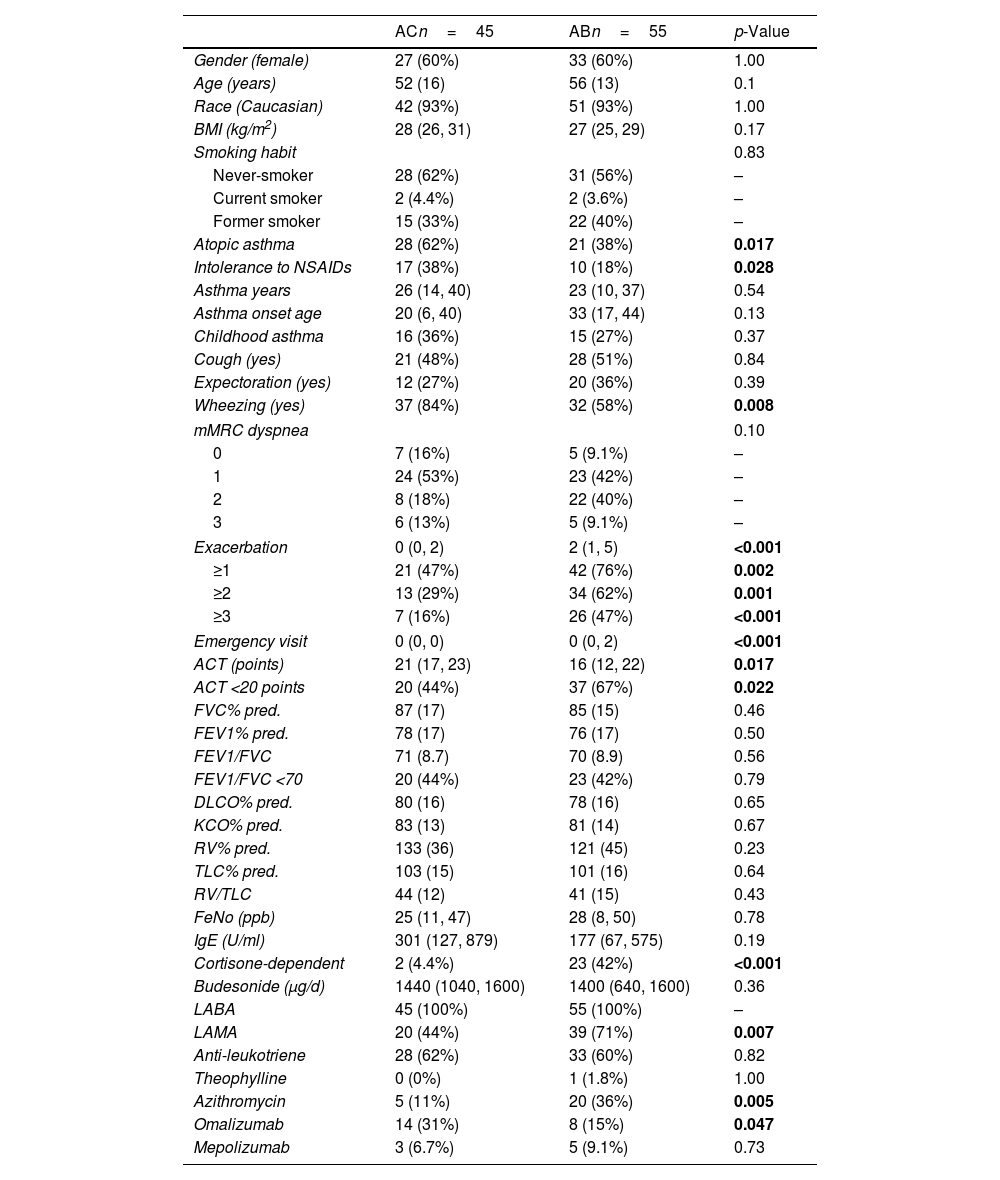
MethodsThe study sample comprised individuals with severe asthma and bronchiectasis (group AB, n=55) and a control population of individuals with severe asthma without bronchiectasis (group AC, n=45). Induced sputum samples were obtained and cell types determined by differential cell count. Proinflammatory and bronchial remodeling cytokines (IL-8, neutrophilic elastase, TGFβ1, VEGF, IFN-γ, TNF-α, and GM-CSF) were analyzed by immunoassay in sputum supernatant.
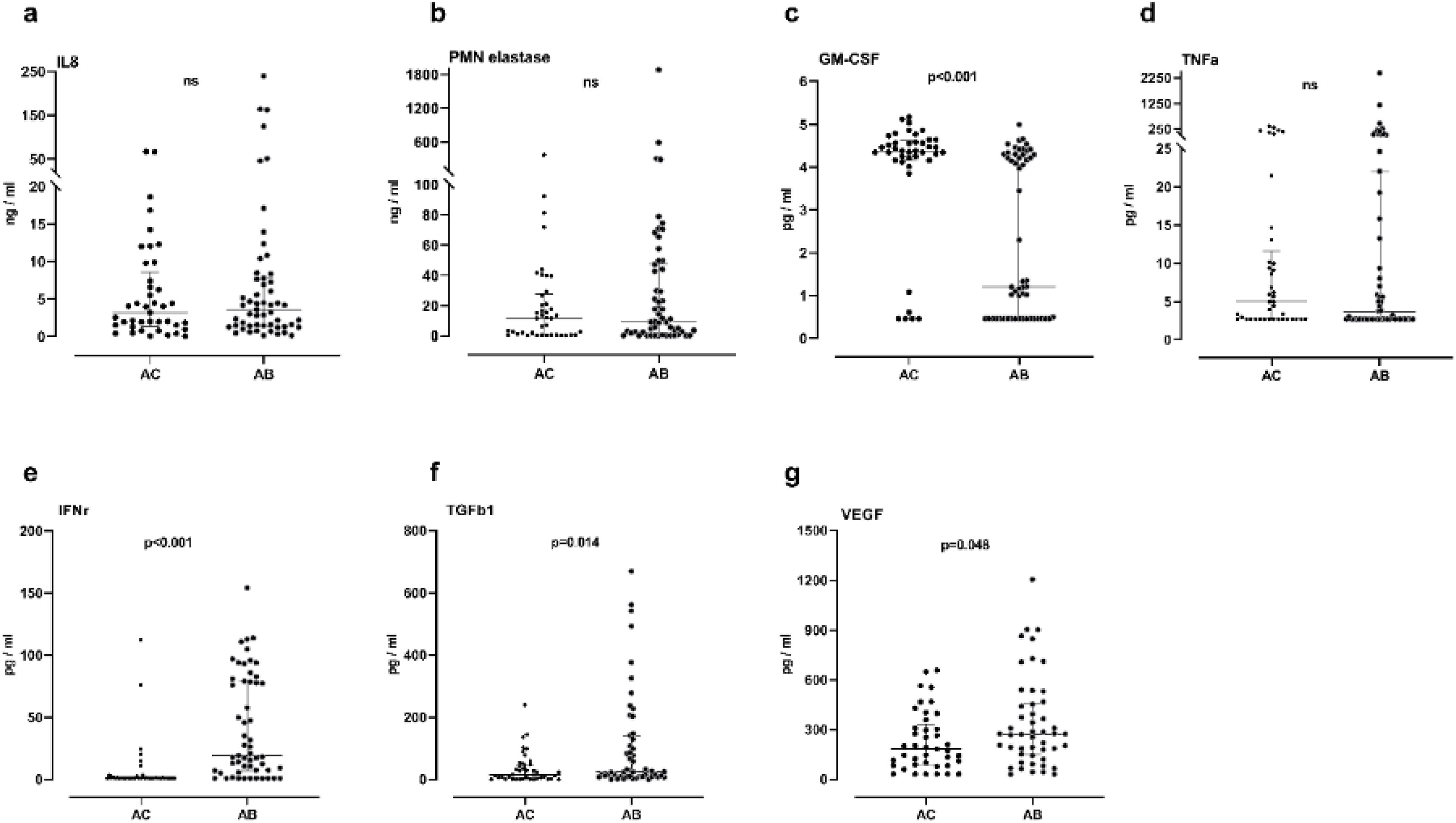
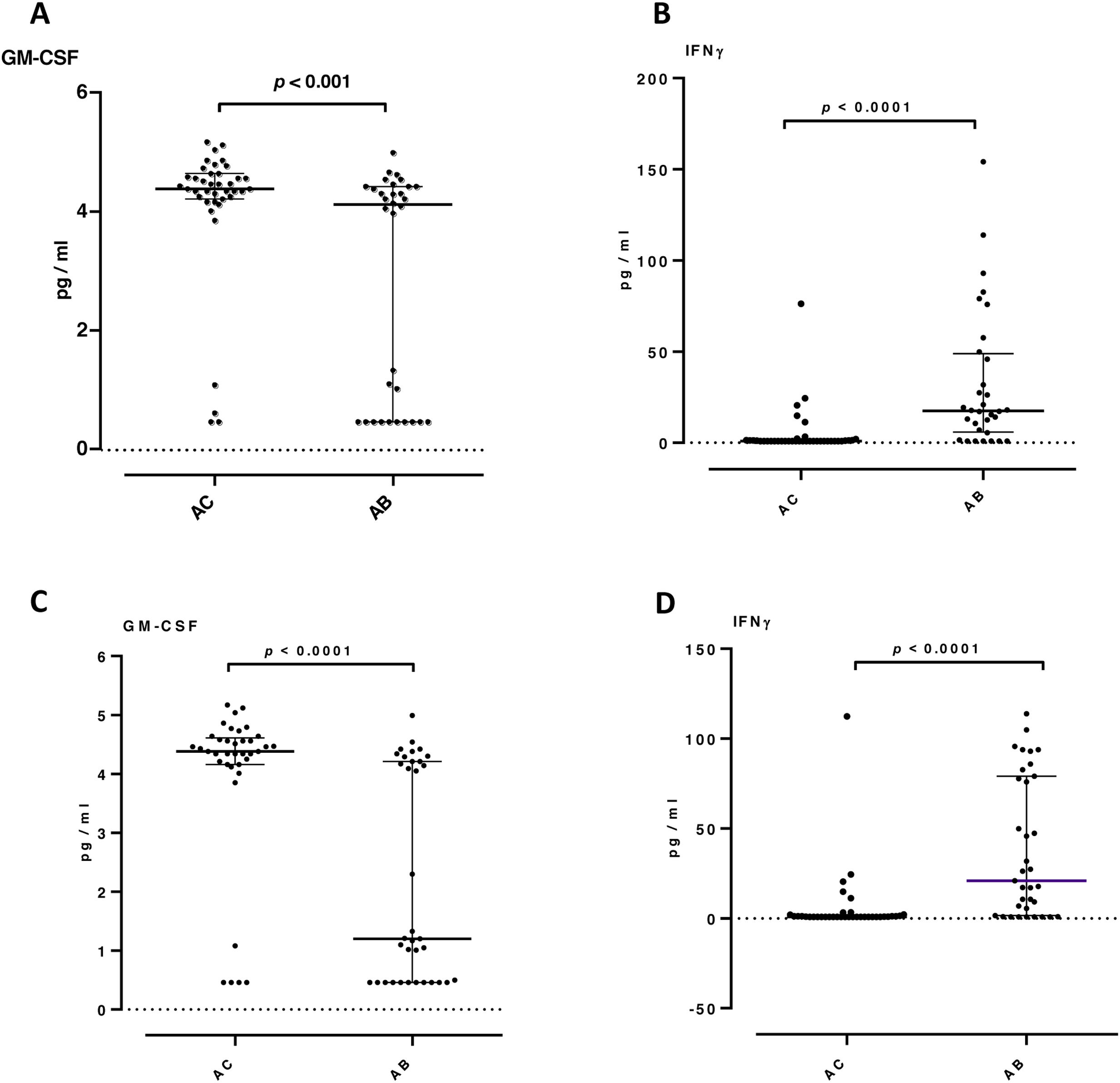
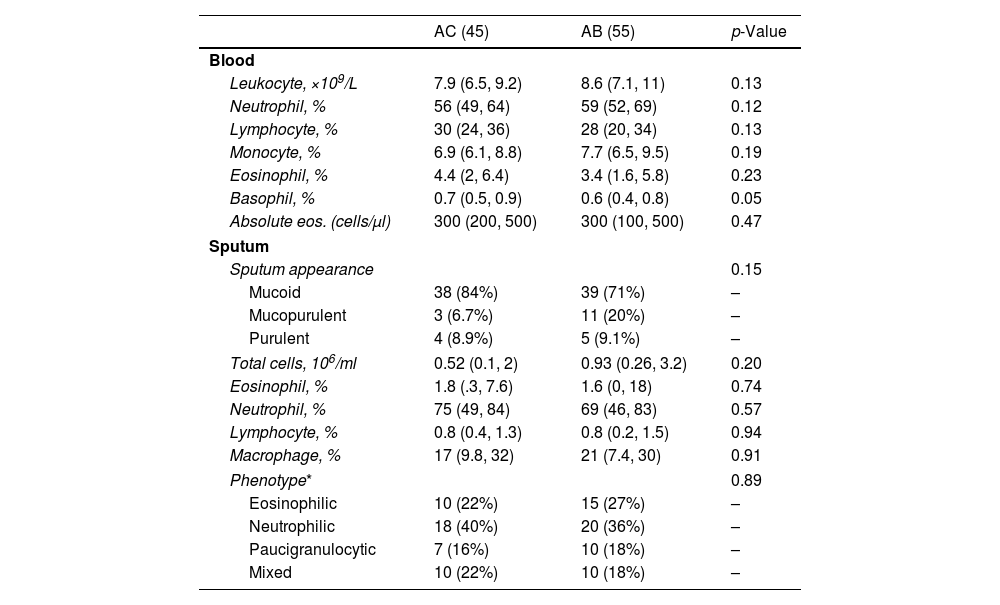
ResultsNeutrophilic inflammation was the primary phenotype in both asthma groups. Higher levels of TGFβ1, VEGF and IFN-γ were observed in asthma patients with bronchiectasis (group AB) than in controls (group AC) (15 vs 24pg/ml, p=0.014; 183 vs 272pg/ml, p=0.048; 0.85 vs 19pg/ml, p<0.001, respectively). Granulocyte-macrophage colony-stimulating factor (GM-CSF) levels were significantly lower in the AB group than in the AC group (1.2 vs 4.4pg/ml, p<0.001). IL-8, neutrophil elastase and TNF-α did not present significant differences between the groups.
ConclusionsRaised levels of TGFβ1 and VEGF cytokines may indicate airway remodeling activation in asthma patients with bronchiectasis. The type of inflammation in asthma patients did not differ according to the presence or absence of bronchiectasis.