Non-tuberculous mycobacteria (NTM) are widely found in the environment and can cause respiratory infections.1 The Mycobacterium avium-intracellulare complex (MAC) is composed of different species, being M. avium and M. intracellulare the most representative members, although in the last years Mycobacterium chimaera has become also relevant since several outbreaks of M. chimaera infections have been reported in patients who underwent cardiac surgery. The heater-cooler devices used in the surgical procedure contained M. chimaera biofilms in their water systems, leading to airborne infections in these patients.2
Over the past decades, respiratory infections produced by MAC have significantly increased, particularly among immunocompromised individuals and patients with lung disease.1 Moreover, MAC isolates have the potential to produce biofilm.3 That is, a microbial community attached to a surface and surrounded by a matrix, which provides protection from the environment, the immune system and antibiotics.4 These communities display different characteristics compared to planktonic forms, such as persistence and increased levels of antibiotic resistance.4–6 Little is known about the characteristics of MAC biofilms,7 and there is no current standardized method for the determination of biofilm formation. Besides, biofilm formation makes MAC respiratory infections even more difficult to treat given that biofilms offer extra protection from antibiotics and other agents.4,5 The antibiotic concentrations required to eradicate mycobacterial biofilms can be a thousand times higher than those needed to inhibit planktonic forms.8,12 Due to the increased antibiotic resistance, these complicated MAC respiratory infections need higher doses of antibiotics in order to improve patient outcome.9,10,13,14 Hence, common antibiotic susceptibility testing may not be useful for those chronic MAC infections where biofilms are involved.8,12 However, there is no unified protocol to assess in vitro biofilm formation in MAC. Therefore, a standardized protocol is needed in order to study the activity of antibiotics and other agents against MAC biofilm. Ideally, standard methods should be easy to perform, affordable, quick to execute and should not require highly specialized equipment.11,15
We report in vitro biofilm formation in MAC clinical isolates by using a protocol based on the adherence to 96-well plates and the crystal violet method. First, we performed the tube method described by Christensen et al.8,12 using 6 M. avium and 6 M. intracellulare clinical isolates obtained from the Microbiology Department of the Hospital Clínic of Barcelona. Briefly, MAC cultures were inoculated in tubes with Middlebrook 7H9 medium (Becton Dickinson, Sparks, MD, USA) at a concentration of 5×106CFU/mL. The tubes were incubated at 37°C for 1 week. After incubation, they were decanted, rinsed with 1×PBS (Gibco, Life Technologies, Belgium) and dried at room temperature. Finally, the tubes were stained with 1% of crystal violet (Comercial Bellés) for 10min. Biofilm formation was considered positive when a visible line appeared. All the 12 MAC isolates showed biofilm formation.
After that, we studied the optimal conditions for the in vitro biofilm formation, based on the adherence to 96-well Plates9,13 and incorporating some modifications previously described by Kumar et al.10 The isolates were grown in tubes containing Middlebrook 7H9 and incubated at 37°C for 1 week. Afterwards, the cultures were centrifuged and the supernatant was discarded. Then, fresh Middlebrook 7H9 was added to each culture. Finally, MAC cultures were homogenized and ajusted to 1×107CFU/mL (CrystalSpec™, Becton Dickinson).
Biofilm formation was determined by seeding 200μL (1×107CFU/mL) of the corresponding MAC culture in 96-well non-treated polystyrene plates (Thermo Fisher Scientific, MA, USA). Negative controls containing Middlebrook 7H9 were also included. The following temperatures and times of incubation were tested, 37°C and 42°C for 3 and 4 weeks each. To minimize evaporation, the plates were covered with a lid. All the experiments were performed in duplicate.
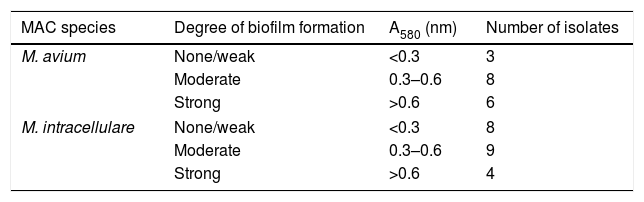
For the biofilm determination, the supernatant of the plates was discarded and each well was rinsed with 1xPBS. The plates were dried at 60°C for 1h and the wells were stained with 1% crystal violet and incubated at room temperature for 10min. Each well was rinsed with 1xPBS and dried at 60°C for 2h. Finally, 33% acetic acid (Vidra Foc, Barcelona, Spain) was added to solubilize the biofilm. The OD was calculated by determining the A580 with a microplate spectrophotometer (Bio Tek, VT, USA). The wells with only Middlebrook 7H9 were used as blanks and their mean A580 values were subtracted from wells containing biofilm. The A580 readings were classified into three categories of biofilm formation: <0.3nm: none/weak; 0.3–0.6nm: moderate; >0.6nm: strong. The optimal conditions for biofilm formation in M. avium were incubation at 42°C for 4 weeks and at 37°C for 4 weeks in M. intracellulare. Finally, this protocol, with the optimal incubation conditions, was performed in a total of 17 M. avium isolates and 21 M. intracellulare clinical isolates.
Slight non-significant differences between M. avium and M. intracellulare were observed, producing M. avium stronger biofilm than M. intracellulare. Thus, 82% of M. avium and 62% of M. intracellulare formed moderate to strong biofilm. On the contrary, 18% of M. avium and 38% of M. intracellulare produced none/weak biofilm, respectively (Table 1).
The results of this study corroborate that an important proportion of MAC clinical isolates can produce biofilm.10,11,14,15 Hence, the assessment of biofilm production in MAC isolates would contribute to a better management of patients with MAC respiratory infections. It is worthy of note that M. avium appears to produce stronger biofilm in comparison with M. intracellulare. Although further data is needed to confirm this fact, it highlights the importance of identifying MAC isolates to the species level. Moreover, the protocol described probed excellent reproducibility, robustness and was easy to perform. In addition, it can be useful to study the effect of antibiotics, alone and in combination, against MAC biofilm.
Summarizing, we determined that a significant number of MAC clinical isolates can produce biofilm. Moreover, the method described showed excellent reproducibility and could be easily applied to screen for MAC biofilm production. This is of great interest given the important role that biofilms play in MAC respiratory infections. Further understanding of MAC biofilm would result in improved patient management.
Ethical statementEthical approval was received from the Ethical Committee of the Hospital Clínic de Barcelona (Barcelona, Spain) [HCB/2016/0344].
FundingThis work was supported by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, co-financed by the European Regional Development Fund (ERDF) ‘A way to achieve Europe’, the Spanish Ministry of Health (grant no. PI16/01047), Planes Nacionales de I+D+i 2008-2011/2013-2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI) (RD16/0016/0010) co-financed by European Development Regional Fund (ERDF) “A way to achieve Europe” and operative program Intelligent Growth 2014-2020. This study was also supported by grant 2017SGR809 from the Departament d’Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya and by a grant from Fundació La Marató de TV3 (grant no. 201816-10). E.P-B received a grant from the Universitat de Barcelona (UB), Ajut de Personal Investigador en Formació (APIF-UB).
Conflict of interestThe authors declare that they have no conflict of interest.
The authors belong to the Study Group of Mycobacterial Infections (GEIM) of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC), the Spanish Network for the Research in Infectious Diseases (REIPI) and the research team awarded for quality control by Agència de Gestió d’Ajuts Universitaris i de Recerca [AGAUR, 2017SGR809]. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. In addition, we are deeply grateful to Donna Pringle for her help with the English redaction.