Immunoglobulin G4-related disease (IgG4-RD) is a recently recognized systemic autoimmune process that brings together a series of diseases that share certain pathological, serological, and clinical characteristics.1 Although it most often affects the pancreas, salivary glands and lymph nodes, almost any structure of the body can be involved. Isolated pulmonary involvement is rare,2 yet the greatest range of clinical and radiological presentations occurs in the lung. IgG4-related lung disease can manifest in the form of bronchovascular thickening, pleural thickening, interstitial involvement, solitary pulmonary nodule, or ground glass opacities that sometimes mimic lung cancer, so differential diagnosis is required.1 However, the coexistence of pulmonary IgG4-RD and lung cancer in the same lesion has only rarely been described.3–5
We report the case of a patient diagnosed with lung cancer and mediastinal lymph node involvement who was treated with surgical resection; the pathology study of the lesion also revealed pulmonary IgG4-RD.
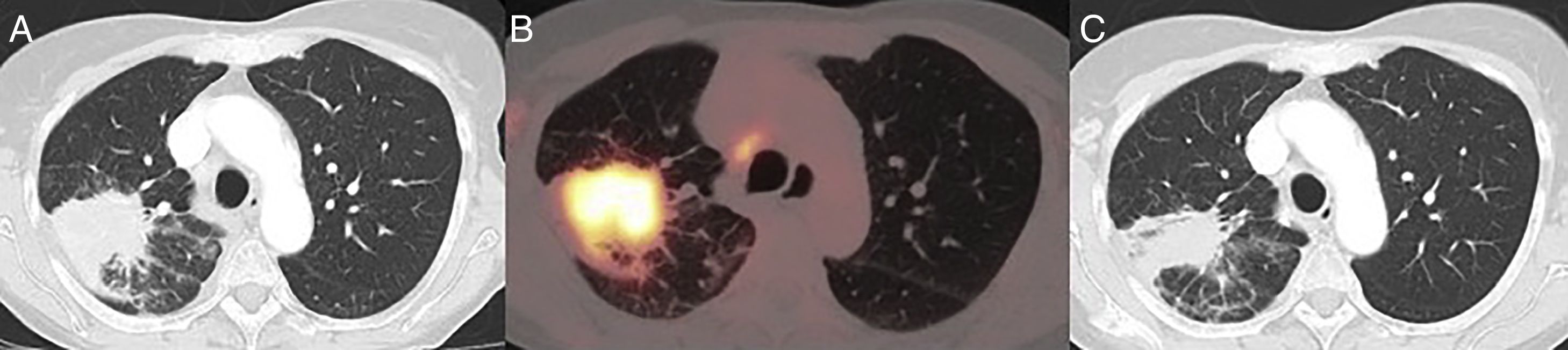
This was 70-year-old woman, former smoker of 50 pack-years, with a clinical history of discoid lupus and atrial fibrillation, who was referred to our department due to an incidental finding of a lung mass on chest X-ray. The patient did not report any respiratory symptoms. Computed tomography (CT) showed a mass with spiculated borders, measuring 6cm, contiguous with the parietal pleura, in addition to hilar and mediastinal lymphadenopathies measuring 10mm (Fig. 1A). Positron emission tomography showed an increased accumulation of fluordeoxyglucose-F18 in the mass with a SUVmax of 9.2 (Fig. 1B), in addition to hypermetabolic lymphadenopathies in the right upper (SUVmax 4) and lower (SUVmax 6.7) paratracheal, subcarinal (SUVmax 5.4) and right pulmonary hilar (SUVmax 4.4) regions. Endobronchial ultrasound biopsy was obtained from the ipso and contralateral hilar and mediastinal lymphadenopathies: the subcarinal mass was positive for metastatic adenocarcinoma (TTF1+). Magnetic resonance imaging ruled out the presence of brain metastasis. Spirometry revealed forced vital capacity 111%, forced expiratory volume in 1s 87%, and diffusing capacity of carbon monoxide 66%. Stair climb test was >22m. After discussion in the multidisciplinary meeting, it was considered a case of locally advanced lung adenocarcinoma with N2 single station involvement, confirmed histologically and potentially resectable (cT3N2M0, stage IIIB), so trimodality treatment was planned with surgical resection and lymphadenectomy, followed by chemotherapy and radiotherapy.6 However, during the week prior to surgery, the patient developed pericarditis and pericardial effusion that forced us to postpone the intervention. The pericardial effusion was drained percutaneously and pericarditis treated with colchicine and nonsteroidal anti-inflammatory drugs. Following resolution of this syndrome, a new CT for re-evaluation was performed that showed a significant decrease in the size of the lesion, which now measured 36mm (Fig. 1C). The patient finally underwent surgery, with right upper lobectomy plus systematic lymphadenectomy via video-assisted thoracoscopy. The pathology report was consistent with adenocarcinoma with a lepidic, acinar and papillary growth pattern, measuring 8mm in diameter, and adenocarcinoma metastases in the subcarinal lymph node (pT1aN2M0, Stage IIIA). The tumor-free lung showed extensive inflammatory lymphoplasmocytic infiltrate with formation of germinal centers, storiform fibrosis, obliterative phlebitis, pulmonary parenchymal atrophy, and alveolar trapping with a marked pneumocytic reaction. The immunohistochemical study showed IgG4-positive cells in more than 10 plasma cells per field. These findings were conclusive for the diagnosis of pulmonary IgG4-RD. The postoperative course of the patient was satisfactory and she was discharged on postoperative day 5 without complications.
(A) CT image on diagnosis, showing a 6cm spiculated lesion in the right upper lobe. (B) Positron emission tomography-CT showing an increased accumulation of fluordeoxyglucose-F18 in the mass with a SUVmax of 9.2. (C) CT image of the re-evaluation CT showing a decreased lesion size after anti-inflammatory therapy.
IgG4-RD has been recently defined as a single autoimmune disease that unites multiple fibroinflammatory diseases previously considered independent entities. It has been described as the new great mimicker, since it imitates the behavior of tumors, inflammatory diseases, and infectious diseases, making it difficult to diagnose and to treat correctly, leading to disease progression.7 The gold standard for diagnosis, apart from manifestations in the affected organ, is the identification of typical histopathological features (lymphoplasmacytic infiltration, storiform fibrosis, and obliterative phlebitis) in the context of a significant infiltration of IgG4-positive plasma cells.1 It is associated in 60%–80% of patients with raised IgG4 levels in serum.8
Some studies suggest that patients with IgG4-RD have an increased risk of developing tumors, including cancer of the lung, pancreas, and colon, and lymphomas.9 However, the coexistence of lung cancer and pulmonary IgG4-RD in a lung mass is extremely rare. In contrast, several cases of IgG4-RD lesions simulating lung tumors have been published.10–13
In the 3 cases published in the literature,3–5 and in our case, the pattern on pathology study was adenocarcinoma.
It is of interest to note that on the diagnostic tests, despite the fact that the tumor component of the mass was only 8mm, the metabolic measurement of the lesion was 5.2cm, suggesting that pulmonary IgG4-RD is associated with an increase in glucose metabolism.10–13 The measurement of early- and late-phase FDG uptake can be useful for differentiating benign and malignant lung lesions. Decreased late-phase uptake of radiomarker has been described in inflammatory processes.14
Most recommendations advocate early treatment of IgG4-RD with immunosuppressive therapy. However, spontaneous resolution of lung disease has also been reported.15 In the case described above, the pulmonary lesion initially measured 60mm, but its size reduced considerably following anti-inflammatory therapy.
We believe that, although lung cancer must be included in the differential diagnosis of pulmonary IgG4-RD, the possibility that both diseases coexist must be considered, so we propose a comprehensive evaluation of patients with suspected IgG4-RD that includes histological confirmation.
Please cite this article as: Gómez Hernández MT, Alvarado IR, Novoa N, Jiménez López MF. Enfermedad pulmonar relacionada con inmunoglobulina G4 como hallazgo incidental tras resección quirúrgica de carcinoma pulmonar. Arch Bronconeumol. 2019;55:276–278.