At the time of writing this document the entire planet is facing the pandemic caused by the SARS-CoV-2 virus. Contrary to the initial thoughts that most infected patients had symptoms, according to data from China collected in April 2020, at the time of the diagnosis, up to 80% of those confirmed to have the disease are asymptomatic, becoming an important source of contagion.1–3 Pan et al. reported that health workers in Wuhan (China) had a significantly higher risk of becoming infected (daily confirmed case rate in local health care workers was 130.5 per million while in the general population it was 41.5 per million).4
In this light, the scientific community is discussing the use of chemoprophylaxis in people at higher risk of infection using several alternatives including antimalarials (chloroquine or hydroxychloroquine) and antiretrovirals (lopinavir-ritonavir).5–10 There is an increased interest in use of chloroquine and hydroxychloroquine, two medications that have experimentally shown to have antiviral capabilities and prophylactic potential.11,12 Lee et al. recently reported good results in an observational study in South Korea after a large COVID-19 exposure event in a hospital. At the end of quarantine, after receiving post-exposure prophylaxis with hydroxychloroquine (400mg daily for 14 days) all follow-up PCR tests were negative in 211 individuals exposed to the index case.13 Certain countries have already adopted chemoprophylaxis schemes; on March 22nd, 2020, the Indian Council of Medical Research's National Task Force for COVID-19 issued a national recommendation to use hydroxychloroquine for prophylaxis against SARS-CoV-2 infection (400mg twice a day on day 1, followed by 400mg once weekly for 7 weeks).14
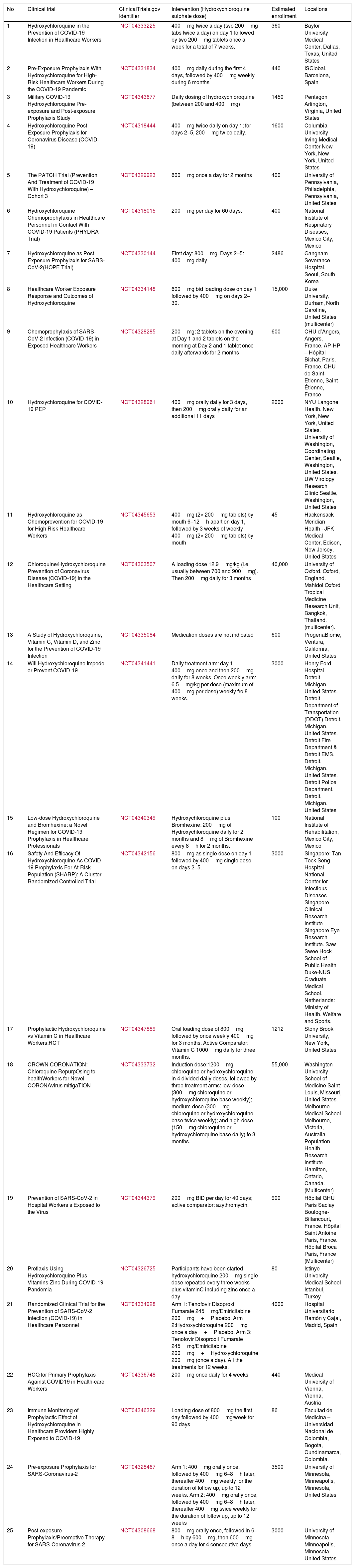
We conducted a search (updated to April 15th, 2020) on the website https://clinicaltrials.gov/. Using the keywords COVID-19 and hydroxychloroquine we found a total of 90 projects registered. Twenty-five of those projects included the use of prophylaxis in non-infected population (Table 1)
Clinical studies about chemoprophylaxis for coronavirus disease (COVID-19) registered on ClinicalTrials.gov (April 15th, 2020).
| No | Clinical trial | ClinicalTrials.gov Identifier | Intervention (Hydroxychloroquine sulphate dose) | Estimated enrollment | Locations |
|---|---|---|---|---|---|
| 1 | Hydroxychloroquine in the Prevention of COVID-19 Infection in Healthcare Workers | NCT04333225 | 400mg twice a day (two 200mg tabs twice a day) on day 1 followed by two 200mg tablets once a week for a total of 7 weeks. | 360 | Baylor University Medical Center, Dallas, Texas, United States |
| 2 | Pre-Exposure Prophylaxis With Hydroxychloroquine for High-Risk Healthcare Workers During the COVID-19 Pandemic | NCT04331834 | 400mg daily during the first 4 days, followed by 400mg weekly during 6 months | 440 | ISGlobal, Barcelona, Spain |
| 3 | Military COVID-19 Hydroxychloroquine Pre-exposure and Post-exposure Prophylaxis Study | NCT04343677 | Daily dosing of hydroxychloroquine (between 200 and 400mg) | 1450 | Pentagon Arlington, Virginia, United States |
| 4 | Hydroxychloroquine Post Exposure Prophylaxis for Coronavirus Disease (COVID-19) | NCT04318444 | 400mg twice daily on day 1; for days 2–5, 200mg twice daily. | 1600 | Columbia University Irving Medical Center New York, New York, United States |
| 5 | The PATCH Trial (Prevention And Treatment of COVID-19 With Hydroxychloroquine) – Cohort 3 | NCT04329923 | 600mg once a day for 2 months | 400 | University of Pennsylvania, Philadelphia, Pennsylvania, United States |
| 6 | Hydroxychloroquine Chemoprophylaxis in Healthcare Personnel in Contact With COVID-19 Patients (PHYDRA Trial) | NCT04318015 | 200mg per day for 60 days. | 400 | National Institute of Respiratory Diseases, Mexico City, Mexico |
| 7 | Hydroxychloroquine as Post Exposure Prophylaxis for SARS-CoV-2(HOPE Trial) | NCT04330144 | First day: 800mg. Days 2–5: 400mg daily | 2486 | Gangnam Severance Hospital, Seoul, South Korea |
| 8 | Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine | NCT04334148 | 600mg bid loading dose on day 1 followed by 400mg on days 2–30. | 15,000 | Duke University, Durham, North Caroline, United States (multicenter) |
| 9 | Chemoprophylaxis of SARS-CoV-2 Infection (COVID-19) in Exposed Healthcare Workers | NCT04328285 | 200mg: 2 tablets on the evening at Day 1 and 2 tablets on the morning at Day 2 and 1 tablet once daily afterwards for 2 months | 600 | CHU d’Angers, Angers, France. AP-HP – Hôpital Bichat, Paris, France. CHU de Saint-Etienne, Saint-Étienne, France |
| 10 | Hydroxychloroquine for COVID-19 PEP | NCT04328961 | 400mg orally daily for 3 days, then 200mg orally daily for an additional 11 days | 2000 | NYU Langone Health, New York, New York, United States. University of Washington, Coordinating Center, Seattle, Washington, United States. UW Virology Research Clinic Seattle, Washington, United States |
| 11 | Hydroxychloroquine as Chemoprevention for COVID-19 for High Risk Healthcare Workers | NCT04345653 | 400mg (2× 200mg tablets) by mouth 6–12h apart on day 1, followed by 3 weeks of weekly 400mg (2× 200mg tablets) by mouth | 45 | Hackensack Meridian Health - JFK Medical Center, Edison, New Jersey, United States |
| 12 | Chloroquine/Hydroxychloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting | NCT04303507 | A loading dose 12.9mg/kg (i.e. usually between 700 and 900mg). Then 200mg daily for 3 months | 40,000 | University of Oxford, Oxford, England. Mahidol Oxford Tropical Medicine Research Unit, Bangkok, Thailand. (multicenter). |
| 13 | A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection | NCT04335084 | Medication doses are not indicated | 600 | ProgenaBiome, Ventura, California, United States |
| 14 | Will Hydroxychloroquine Impede or Prevent COVID-19 | NCT04341441 | Daily treatment arm: day 1, 400mg once and then 200mg daily for 8 weeks. Once weekly arm: 6.5mg/kg per dose (maximum of 400mg per dose) weekly fro 8 weeks. | 3000 | Henry Ford Hospital, Detroit, Michigan, United States. Detroit Department of Transportation (DDOT) Detroit, Michigan, United States. Detroit Fire Department & Detroit EMS, Detroit, Michigan, United States. Detroit Police Department, Detroit, Michigan, United States |
| 15 | Low-dose Hydroxychloroquine and Bromhexine: a Novel Regimen for COVID-19 Prophylaxis in Healthcare Professionals | NCT04340349 | Hydroxychloroquine plus Bromhexine: 200mg of Hydroxychloroquine daily for 2 months and 8mg of Bromhexine every 8h for 2 months. | 100 | National Institute of Rehabilitation, Mexico City, Mexico |
| 16 | Safety And Efficacy Of Hydroxychloroquine As COVID-19 Prophylaxis For At-Risk Population (SHARP): A Cluster Randomized Controlled Trial | NCT04342156 | 800mg as single dose on day 1 followed by 400mg single dose on days 2–5. | 3000 | Singapore: Tan Tock Seng Hospital National Center for Infectious Diseases Singapore Clinical Research Institute Singapore Eye Research Institute. Saw Swee Hock School of Public Health Duke-NUS Graduate Medical School. Netherlands: Ministry of Health, Welfare and Sports. |
| 17 | Prophylactic Hydroxychloroquine vs Vitamin C in Healthcare Workers:RCT | NCT04347889 | Oral loading dose of 800mg followed by once weekly 400mg for 3 months. Active Comparator: Vitamin C 1000mg daily for three months. | 1212 | Stony Brook University, New York, United States |
| 18 | CROWN CORONATION: Chloroquine RepurpOsing to healthWorkers for Novel CORONAvirus mitigaTION | NCT04333732 | Induction dose:1200mg chloroquine or hydroxychloroquine in 4 divided daily doses, followed by three treatment arms: low-dose (300mg chloroquine or hydroxychloroquine base weekly); medium-dose (300mg chloroquine or hydroxychloroquine base twice weekly); and high-dose (150mg chloroquine or hydroxychloroquine base daily) fo 3 months. | 55,000 | Washington University School of Medicine Saint Louis, Missouri, United States. Melbourne Medical School Melbourne, Victoria, Australia. Population Health Research Institute Hamilton, Ontario, Canada. (Multicenter) |
| 19 | Prevention of SARS-CoV-2 in Hospital Workers s Exposed to the Virus | NCT04344379 | 200mg BID per day for 40 days; active comparator: azythromycin. | 900 | Hôpital GHU Paris Saclay Boulogne-Billancourt, France. Hôpital Saint Antoine Paris, France. Hôpital Broca Paris, France (Multicenter) |
| 20 | Proflaxis Using Hydroxychloroquine Plus Vitamins-Zinc During COVID-19 Pandemia | NCT04326725 | Participants have been started hydroxychloroquine 200mg single dose repeated every three weeks plus vitaminC including zinc once a day | 80 | Istinye University Medical School Istanbul, Turkey |
| 21 | Randomized Clinical Trial for the Prevention of SARS-CoV-2 Infection (COVID-19) in Healthcare Personnel | NCT04334928 | Arm 1: Tenofovir Disoproxil Fumarate 245mg/Emtricitabine 200mg+Placebo. Arm 2:Hydroxychloroquine 200mg once a day+Placebo. Arm 3: Tenofovir Disoproxil Fumarate 245mg/Emtricitabine 200mg+Hydroxychloroquine 200mg (once a day). All the treatments for 12 weeks. | 4000 | Hospital Universitario Ramón y Cajal, Madrid, Spain |
| 22 | HCQ for Primary Prophylaxis Against COVID19 in Health-care Workers | NCT04336748 | 200mg once daily for 4 weeks | 440 | Medical University of Vienna, Vienna, Austria |
| 23 | Immune Monitoring of Prophylactic Effect of Hydroxychloroquine in Healthcare Providers Highly Exposed to COVID-19 | NCT04346329 | Loading dose of 800mg the first day followed by 400mg/week for 90 days | 86 | Facultad de Medicina – Universidad Nacional de Colombia, Bogota, Cundinamarca, Colombia. |
| 24 | Pre-exposure Prophylaxis for SARS-Coronavirus-2 | NCT04328467 | Arm 1: 400mg orally once, followed by 400mg 6–8h later, thereafter 400mg weekly for the duration of follow up, up to 12 weeks. Arm 2: 400mg orally once, followed by 400mg 6–8h later, thereafter 400mg twice weekly for the duration of follow up, up to 12 weeks | 3500 | University of Minnesota, Minneapolis, Minnesota, United States |
| 25 | Post-exposure Prophylaxis/Preemptive Therapy for SARS-Coronavirus-2 | NCT04308668 | 800mg orally once, followed in 6–8h by 600mg, then 600mg once a day for 4 consecutive days | 3000 | University of Minnesota, Minneapolis, Minnesota, United States. |
Institutions from 13 countries are leading those projects, 13 of them from the United States, 2 from Mexico, 2 from Spain and 2 from France. Turkey, Colombia, Austria, South Korea, Singapore, United Kingdom, Thailand, Australia and Canada, have institutions leading one project (there are more institutions than projects, because some have shared leadership).
There is a significant variability in the number of participants among the studies. The estimated number of participants to be enrolled ranges between 45 and 55,000 with an average of 5588±13,139.2, and a median of 1212 participants. Only 3 (12%) studies plan to enroll more than 4000 participants; those 3 studies will include 70,000 participants which corresponds to 50.1% of the total potential recruitment of the 25 protocols (NCT04334148, NCT04303507 and NCT04333732).
There is a significant variability among protocols regarding hydroxychloroquine maintenance dose, which will be between 200 and 600mg. The frequency is also highly variable: seventeen protocols will use daily prophylaxis for a period from 4 days to 12 weeks and 9 protocols plan to evaluate weekly use for a period of 3 to 24 weeks. Thirteen (52% of 25) protocols will use an initial loading dose ranging between 400–1400mg taken on the first day. In three other protocols, 2–4 days of loading doses of 400mg/day will be indicated.
19 clinical trials will evaluate pre-exposure prophylaxis and 6 post-exposure prophylaxis. In 9 of the pre-exposure prophylaxis studies and 4 of the post-exposure prophylaxis studies, a loading dose of 800mg of hydroxychloroquine will be started on the first day. In an additional multicenter pre-exposure prophylaxis study, which plans to recruit 15,000 participants (NCT04334148), they will use a higher loading dose on the first day: 1200mg of hydroxychloroquine.
We evaluated the exclusion criteria among protocols by grouping into several possible categories. Most common criteria used by protocols to exclude patients comprised allergies to 4-aminoquinolines (hydroxychloroquine, chloroquine) in 20 studies (80%); retinopathy in 19 (76%); history of a prolonged QT syndrome, use of medications that prolong the QT/QTc interval or risk factors for torsades de pointe in 17 (68%); nephropathy in 16 (64%); pregnancy or breastfeeding in 14 (56%); concomitant use of other medications with potential pharmacological interaction with 4-aminoquinolines in 13 (52%); liver disease in 13 (52%); psoriasis or porphyria in 11 (44%) and glucose-6-phosphate dehydrogenase deficiency in 9 protocols (36%).
We used only clinicaltrials.gov to search the trials, which is a weakness of our study, because several protocols may be lost (those registered on https://www.clinicaltrialsregister.eu/, http://www.chictr.org.cn/index.aspx or https://apps.who.int/trialsearch/). However, due to the rapidity of the events related to the COVID-19 pandemic, we consider that the initial publication of the review including only those registered on clinicaltrials.gov is warranted, in order to give clinicians a preliminary picture.
According to the World Health Organization, as of April 15th, 2020, there are almost 2 million confirmed cases of SARS-CoV-2 virus in 213 countries and territories, but this is a underestimation, because as mentioned, around 80% of the infected people could be asymptomatic and go undetected.4 A high infection rate among health care workers would not only exacerbate the impending shortage of health care facilities and health personnel but would also increase the possibility of a more widespread dissemination.5 However, the current scientific evidence is still not conclusive for institutions and governments to adopt a general recommendation regarding the prophylactic use of hydroxychloroquine chemoprophylaxis in healthcare workers.
Chloroquine and its derivatives (e.g., hydroxychloroquine) have been used for malaria and autoimmune rheumatic diseases for almost 80 years, and both the data from the literature and the experience of clinicians show a low incidence of side effects, which are generally mild to moderate. Retinal toxicity, a serious effect, is related to long-term cumulative dose, and is rarely seen in short-term use (i.e. several weeks).9,15
These arguments perhaps tip the balance in favor of using prophylaxis for SARS-CoV-2 virus infection, as long as they are not contraindicated. Nonetheless we are yet to know the results of these clinical trials.