A 75-year-old white male with a history of coronary artery disease, paroxysmal atrial fibrillation and stable chronic heart failure, presented with progressive shortness of breath without orthopnoea or coughing, not responsive to increasing the dose of his diuretics. Chest X-ray and CT showed compression atelectasis of the left lung, caused by a moderate left-sided pleural effusion (140000 RBCs; 1840 WBC [76% macrophages, 15% lymphocytes]; LDH 8488IU/L; protein 40.4g/L, glucose <20mg/dl). No malignant cells were found in either sample; all cultures (including mycobacterial PCRs) were negative.
Blood analysis showed normal kidney function, elevated C-reactive protein (43.9mg/L) and leucocytosis (7.53×109/L), no anaemia, mildly decreased serum albumin (26.9g/L), and elevated lactate dehydrogenase (549IU/L). Auto-immune screening showed a positive antinuclear antigen (1/320) with positive anti-mitochondrial antibody (M2-3E[BPO]). However, no auto-immune disorders could be identified. Hepatitis B and C serology was negative. Epstein–Barr virus (EBV) IgG was 404U/ml. HIV serology was negative.
A smaller effusion at the right side was also present. No enlarged mediastinal or axillary lymph nodes were found. The pleural effusion quickly returned, necessitating a second needle aspiration. A pleuroscopy with pleural biopsies, followed by pleurodesis was performed. Over the next 2 months, the limited pleural effusion that had been present at the right side increased. Fluid analysis again showed exudate (636 RBCs; 401 WBCs; 53% macrophages, 24% lymphocytes; 13.5% abnormal lymphoid cells; LDH 2873IU/L).
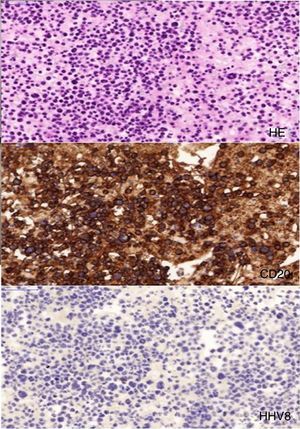
Flow cytometry followed by immunostaining showed a large monoclonal B-cell population with presence of CD20, CD30 (though weak), CD45, MUM1, and BCL-6 markers, and presence of immunoglobulin heavy chain rearrangement, suggestive of diffuse large B-cell lymphoma (DLBCL, Fig. 1). CD3, CD10, CD15, EBV, and HHV-8 immunostaining were negative. Diagnosis of an HHV-8-unrelated primary pleural diffuse large B-cell lymphoma was made. Retrospectively, we managed to identify this lymphoma in the samples of the left-side pleural effusion. Our patient refused treatment and was lost to follow-up soon after.
Primary effusion lymphoma (PEL), also called body-cavity-based-lymphoma (BCBL), is a rare type of B-cell non-Hodgkin's lymphoma confined to the body cavities, thus presenting as a pleural, pericardial or peritoneal (ascites) serous effusion. A detectable solid tumour mass is never present. It was first described by Nador in 1996.1 According to the WHO classification of tumours of hematopoietic and lymphoid tissue, PEL is considered a variant of diffuse large B-cell lymphoma.2
Ichinohasama proposed a classification that divides primary effusion lymphoma into three subtypes3:
- (1)
classic PEL, related to human herpes virus 8-infection, usually in HIV-seropositive patients (but also in areas with high HHV-8-prevalence, such as the Mediterranean). The role of HHV-8 in lymphomagenesis has also been recognised in Kaposi's sarcoma, multicentric Castleman's disease and plasmablastic oral cavity lymphoma, but the exact mechanism in PEL is not entirely understood.2 Prognosis is very poor with a median survival of 3–4 months.
- (2)
PEL-like lymphoma, as in our case, which is HHV-8-unrelated. Typical (pan-) B-cell markers, such as CD19, CD20, CD79a, and clonal immunoglobulin heavy chain (IgH) rearrangement are present, whilst activation and plasma cell markers, such as CD30, CD38, CD45, CD138 are not present, in contrast to classic PEL. Prognosis is less unfavourable. A mean survival of 8.5–10.1 months and a 1-year survival of 35% have been reported.4
- (3)
Burkitt-like lymphoma or extranodal Burkitt lymphoma is characterised by presence of c-myc gene rearrangement, which is absent in classic PEL and PEL-like lymphoma.
HHV-8-related PEL patients typically are younger (mean age 42.5 years), compared to HHV-8-unrelated PEL-like lymphoma (mean age 81.5 years), resembling the distribution in Kaposi's sarcoma, found early in HIV-infection (i.e. younger patients) and in HIV-negative older patients. In general, PEL-like lymphoma seems to be a disease of the elderly, and is often associated with states of chronic inflammation or fluid overload, such as liver cirrhosis or heart failure (Ardiguzel reported an association with HCV in 41.2% of cases4). Many cases may be missed, as this entity is not very well known and (bilateral) pleural effusion may be attributed to heart failure itself instead.
Because of the small number of cases, no standard treatment has been determined. Patients have usually been treated with chemotherapy consisting of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), in combination with rituximab, as HHV-8-unrelated PEL-like lymphoma usually expresses the CD20 B-cell marker; this is the standard regimen for systemic B-cell lymphoma. Therapy with rituximab as a single agent in older patients with lymphoma expressing CD20 may be an alternative, as Xiao reported a case of persistent remission for 5 years with rituximab alone.5 Spontaneous regression after pleurodesis or fluid drainage has been reported.3
We noted a very high pleural to serum LDH ratio, which is, although not specific, very suggestive of pleural lymphoma. We were able to find 7 more cases that reported pleural and serum LDH. Our finding was confirmed in each of these cases.
We would like to thank Jasper Bruyneel for preparing the image of the lymphoma staining and Els De Droogh, Danny Galdermans and Lieven Bedert for their comments.
Please cite this article as: Raskin J, Slabbynck H, Beel K. Derrame linfomatoso primario bilateral, no asociado al herpesvirus humano 8, en un paciente con hipervolemia crónica. Arch Bronconeumol. 2016;52:492–493.