Interpreting and evaluating the importance of patient-ventilator asynchronies in non-invasive mechanical ventilation (NIMV) is an extremely difficult task. Some of the experiences published in the literature suggest that some asynchronies detected in clinical practice are directly induced by the ventilator, as muscles respond to mechanical stimuli. For example, the phenomenon known as “reverse triggering”1 has been described in profoundly sedated adult patients receiving invasive ventilation and may be a new form of diaphragmatic neuromechanical coupling, induced by a reflex mediated by adaptation of the stretch receptors during inspiration (Hering-Breuer reflex). This phenomenon has only been described in sedated patients receiving invasive ventilation, and to date no cases of muscle response to mechanical stimuli have been described in patients receiving NIMV.
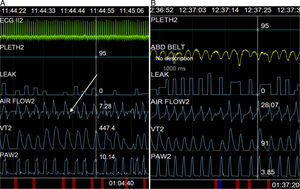
We report the case of a 62-year-old woman with amyotrophic lateral sclerosis, with predominant upper neuron involvement and significant hyperreflexia. NIMV was indicated due to forced vital capacity below 50% predicted value, mild hypercapnia (PaCO2 46mmHg) and incipient intolerance to a decubitus position. Titration began with a nasal interface and chinstrap for periods of 1–2h on consecutive days with a Lumis® 150 pressure ventilator (ResMed, North Ryde, Australia). Parameters at the end of the first session were: IPAP 18cmH2O, EPAP 5cmH2O, rise time 150ms, Timin 0.6s and Timax 1.5s, triggering and cycling settings at mean values. Unintentional leak was maintained at acceptable values after a chinstrap was placed (less than 10l/min overall), and breathing rate was around 18–20bpm. During real time monitoring of pressure-time and flow-time curves, deflection was observed at the start of flow-time waveform expiration, despite good initial tolerance (Fig. 1A).
Since deflection occurred at the start of expiration, with persistent exertion during this phase (premature cycling asynchrony2), the cycling setting was modified, prolonging rise time to 250ms (in order to delay maximum flow, and thus, cycling), and Timin of 0.8 was superimposed, but the abnormality persisted. Finally, while previous rise time values were maintained without superimposing the time criterion, the deceleration ramp was modified from inspiration to expiration, and was set at 250ms, with subsequent resolution of the disorder (Fig. 1B).
The abnormality could be resolved only by modifying the descent time in this patient with marked hyperreflexia, suggesting that the visible alteration in flow–time curves might be due to an automatic response of the patient's respiratory system. This is similar to the situation described by Akoumianaki et al.,1 although these authors described the phenomenon in patients receiving sedation and relaxation. Instead of being a chest expansion reflex, the response appears to be associated more with a deflation reflex that remains relatively constant from cycle to cycle.
Not all ventilator models record deceleration. To date, its use in clinical practice has not been reported in the literature, although, theoretically at least, it should improve tolerance to the sudden flow inversion that occurs during the transition from inspiration to expiration. Most of the time this flow inversion, that may be as high as 80lx′ [although in our patient it was around 60lx′ (20–40lx′)] does not produce any symptoms, but some patients might report discomfort. In our patient, the flow inversion appeared to trigger an automatic respiratory response, intensified by concomitant generalized hyperreflexia. Amyotrophic lateral sclerosis may present in different forms, not only in terms of muscle topography, but also in the degree of spasticity and hyperreflexia. Thus, predominant lower motor neuron involvement produces weakness and atrophy, while upper motor neuron involvement produces basically spastic hypertonia and hyperreflexia that can affect any muscle group. Reflecting findings made first in animal models and later in patients,3 our patient's diaphragm may have been activated due to deflation in the presence of vagal nerve integrity. This anomaly can also be observed on spirometry in forced expiratory maneuvers, when cough is induced by maximum expiration (Fontana reflex).4
In conclusion, patient-ventilator asynchrony observed in our patient does not appear to be the same as cases previously described in the literature, since it does not meet the characteristics of premature cycling (it is not modified by prolonging inspiratory time or cycling settings) or reverse triggering (the cycle before the asynchrony is not controlled). For this reason, diaphragmatic activation during transition to expiration may be a plausible interpretation that would explain the persistence of asynchrony and improvement with progressive depressurization.
Please cite this article as: Galdeano M, Luján M. Reflejo de Hering-Breuer y ventilación mecánica no invasiva ¿también durante la espiración? Arch Bronconeumol. 2016;52:618–619.