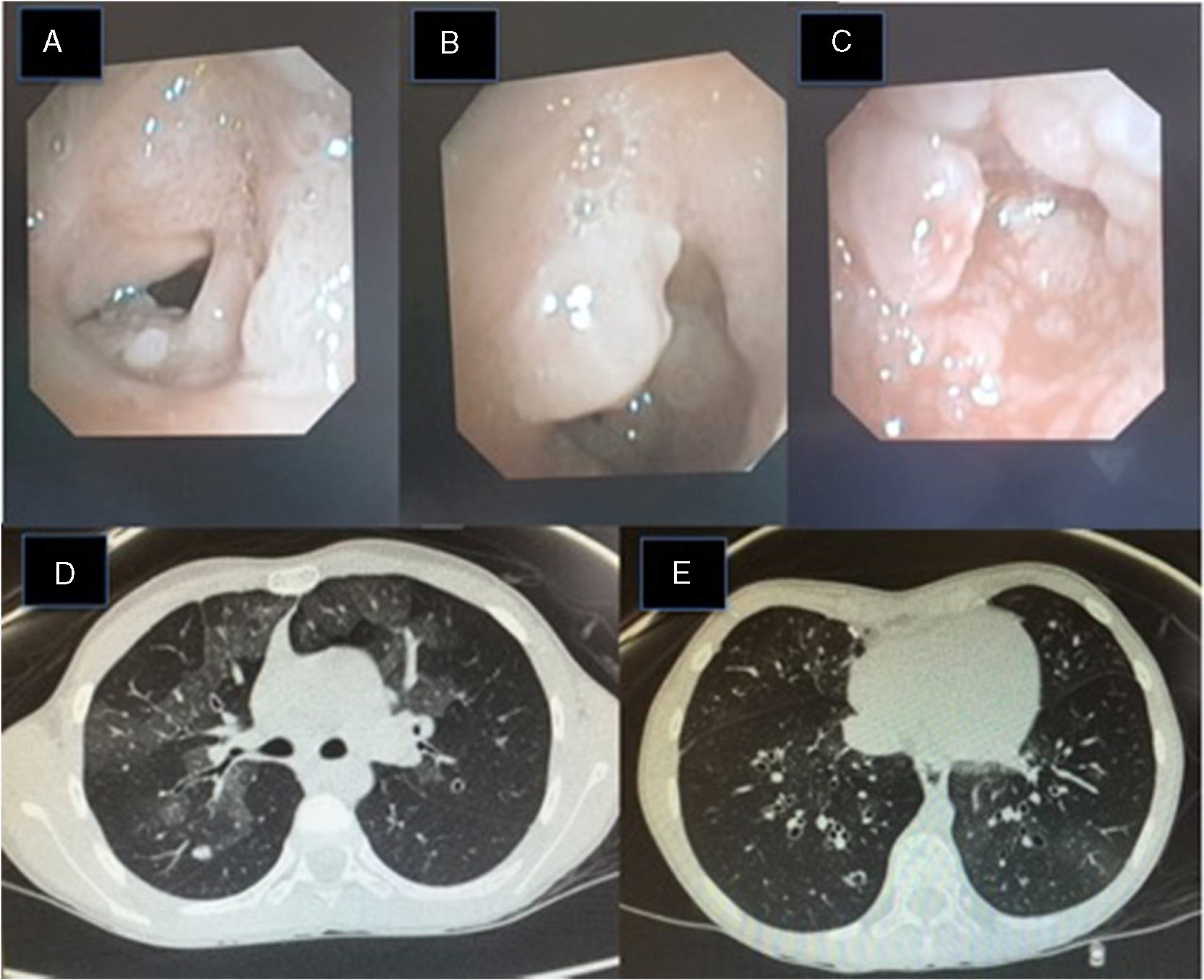
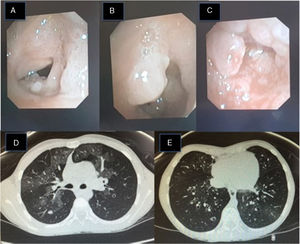
Recurrent laryngeal papillomatosis (RLP) is characterized by the recurrent growth of papillomas in the respiratory tract. It is caused by the human papilloma virus (HPV), and serotypes 6 and 11, with low oncogenic risk, account for almost 90% of cases; other serotypes (16, 18, 31, 33) are rare.1 Its incidence is unknown; in the United States, an estimated 4.3:100000 children and 1.8:100000 adults are affected.2 Presentation occurs with a bimodal distribution, with both a juvenile and adult form.3 The juvenile form is the main cause of laryngeal tumors in children and the second cause of spasmodic dysphonia, which is the most frequent guiding symptom,4 but given its non-specific clinical picture, diagnosis may be delayed for up to a year.5 Infection with HPV 11 and diagnosis before 3 years of age are major risk factors for the development of severe forms, and these factors are associated with more recurrences.3 In the most aggressive forms, the airway may be so severely compromised as to require tracheostomy. The clinical course is unpredictable, and the clinical spectrum varies from progressive and spontaneous remission, through recurrences and endobronchial dissemination of papillomatous lesions6 to malignant conversion (2%).6 It is associated with high morbidity and a heavy psychosocial impact on patients and family members, and generates significant healthcare expenditure.7 There is currently no curative treatment.3,4 The mainstay of treatment is surgery with repeated endoscopies to clear obstructions from the airway.3,4 Numerous adjuvant medical treatments have been tested, but with limited success.8 The most commonly used are intralesional cidofovir9 and systemic interferon.10 The subglottic dissemination of papillomatous lesions worsens prognosis considerably3 by complicating the surgical approach.3 According to the literature, nebulized cidofovir has been used in cases of distal dissemination,11,12 and has been well tolerated and shown good pathological response11,12 (Fig. 1).
(A–C) Fiberoptic bronchoscopy image: pearly skin lesions in the posterior third of the vocal cords and vestibular folds of the glottis, extending to the trachea, causing 90% stenosis of the tracheal lumen. These lesions bleed easily. (D) Lung HRCT image: bilateral pulmonary ground glass pattern and a 6mm nodule in the posterior segment of the right upper lobe. (E) Lung HRCT: cylindrical bronchial dilation with slight thickening of bronchial walls.
We report our experience with the compassionate use of nebulized cidofovir in 2 pediatric patients with RLP with endobronchial dissemination, who responded poorly with significant side effects. Consent for publication of both cases was obtained from the patients’ legal representatives.
Clinical case 1: Girl with a personal history of prematurity and orotracheal intubation at birth. Diagnosed with HPV RLP at 6–9 months of age due to progressive inspiratory stridor. A tracheostomy was placed 3 months after diagnosis. Since then, she has needed monthly surgical interventions for resection of lesions and has received adjuvant treatment with subcutaneous interferon alfa, intralesional cidofovir, oral 3-indolcarbinol, and tetravalent anti-HPV vaccine (GardasilTM). At the age of 3 years, during fiberoptic bronchoscopy, a papillomatous lesion was observed in the right main bronchus. Treatment began with nebulized cidofovir (4ml at a concentration of 10mg/ml, 3 times per week). After administration of the fifth dose, she consulted due to frank hemoptysis and desaturation, requiring hospital admission for control of symptoms. Nebulized cidofovir was suspended, and since then she has not presented any more bleeding episodes.
Clinical case 2: Girl diagnosed with HPV RLP at 11–18 months of age, requiring a tracheostomy to maintain airway patency, which she still requires. Since diagnosis, she has needed fortnightly/monthly surgeries, and has received treatment with intralesional and systemic cidofovir, subcutaneous interferon alpha, and tetravalent anti-HPV vaccine (GardasilTM). At the age of 8 years, she consulted due to progressive respiratory difficulty, and fiberoptic bronchoscopy revealed papillomatous lesions in the trachea (Fig. 1). We decided to start nebulized cidofovir at a lower concentration, given the previous experience (4ml at a concentration of 5mg/ml, 3 times/week), with good clinical response (minimal residual papillomatous lesions 6 months later). Nine months after starting treatment, she developed constitutional syndrome, hypoxemia, and increased breathing difficulty. HRCT of the lung showed patchy focal areas of ground glass density and bilateral bibasal cylindrical bronchiectasis (Fig. 1), findings that, along with the clinical picture, helped establish the diagnosis of bronchiolitis obliterans organizing pneumonia (BOOP). The nebulized cidofovir was discontinued and corticosteroids started at 2mg/kg, enabling withdrawal of oxygen and leading to clinical improvement. HRCT follow-up of the lung revealed radiological improvement, both in the ground glass pattern and the cylindrical bronchiectasis. Currently, corticosteroids have been suspended, and the patient is receiving aerosol therapy and respiratory physiotherapy for her condition.
In conclusion, the therapeutic management of RLP is a challenge, given the lack of effective therapeutic alternatives, and the significant adverse effects of the available options. In the absence of controlled studies in significant cohorts, the evidence published to date11,12 does not support the use of nebulized cidofovir in patients with RLP, as its side effects are potentially serious, and its effectiveness is doubtful.
The publication of experience of the efficacy and safety of “off-label” treatments in this rare disease could help optimize therapeutic decision-making in daily clinical practice, improving the management and quality of life of these patients.
Please cite this article as: Sánchez-Moreno P, Falcón-Neyra L, Neth O, Delgado Pecellín I. Hemoptisis y bronquiolitis obliterante en niños con papilomatosis laríngea recurrente: reacciones adversas al cidofovir nebulizado. Arch Bronconeumol. 2019;55:386–387.