Hemoptysis is the expectoration of blood originating in the lower respiratory tract.1 It can be caused by diseases of the respiratory tract, lung parenchyma or the pulmonary vasculature, but it can also be idiopathic.1 Chronic bronchitis, bronchiectasis and lung cancer are the most common causes in developed countries.1 We report the case of a patient with hemoptysis, attributed to the use of acenocoumarol, which was controlled when acenocumarol was switched to a direct-acting oral anticoagulant (DOAC).
This was a 75-year-old man, former smoker (cumulative consumption: 30 pack-years), with a history of arterial hypertension, diabetes mellitus, dyslipidemia, ischemic stroke in the vertebrobasilar territory without sequelae, angina on exertion, permanent non-valvular atrial fibrillation, anticoagulated with acenocoumarol within the therapeutic range (CHA2DS2-VASc score of 6 points, HAS-BLED 1 point), chronic renal failure due to diabetic nephropathy (creatinine 1.76mg/dl, glomerular filtration rate 38ml/min/1.73m2), transurethral resection of non-invasive, low grade papillary urothelial carcinoma (TaG1), not requiring adjuvant treatment and in complete remission, polymyalgia rheumatica, gout, and operated for varicose veins 5 years previously. In January 2018, he came to the emergency room with a 2-month history of scant bloody sputum, and was admitted to complete the study. Cardiac auscultation revealed arrhythmic heart tones, while the rest of the physical examination was unremarkable. Clinical laboratory tests were significant for INR within the therapeutic anticoagulation range, and no acute changes were observed on chest X-ray. Computed tomography (CT) of the chest showed ground glass infiltrates in both lower lobes and in the middle lobe, which was accompanied by smooth thickening of the interlobular septa. These findings suggested a number of possible diagnoses, including acute pulmonary edema, aspirated blood, and desquamative interstitial pneumonia, and a repeat CT within approximately 3 months was recommended. Fiberoptic bronchoscopy showed that both bronchial trees were patent with normal mucosa, and no hematic remnants were detected at any level. Both microbiology and cytology of the bronchial aspirate were negative. Given these findings, the patient was discharged after restarting treatment with acenocoumarol, which had been suspended during admission.
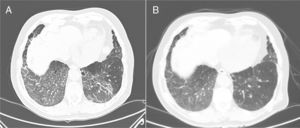
Three months later, the patient reported persistent, although scant, hemoptysis. In the follow-up chest CT, ground glass infiltrate was observed in both lung bases and in the middle lobe, with associated linear thickening of the interlobular septa, which had increased significantly with respect to the previous study (Fig. 1A). In view of these findings, fiberoptic bronchoscopy was repeated and transbronchial biopsy was performed in the right lower lobe, which showed histological changes consistent with organizing pneumonia. In view of the prolonged hemoptysis in the absence of associated endobronchial lesions, and the possibility that it might be associated with acenocoumarol treatment, despite the fact that clinical laboratory tests in the past few months showed INR in the therapeutic anticoagulation range (INR between 2 and 3, with an accumulated time within the therapeutic range of 65.5% and 100% since the last measurement), we decided to discontinue treatment with acenocoumarol and to begin a DOAC the following day, specifically apixaban (its use has been associated with lower rates of bleeding in clinical trials). The dose was 5mg/12h, without dose adjustment, as the patient's glomerular filtration rate was greater than 30ml/min/1.73m2.
(A) CT scan showing ground glass infiltrates in both lung bases and middle lobe, with associated linear thickening of the interlobular septa. (B) Follow-up computed axial tomography, performed 3 months after switching treatment from acenocoumarol to apixaban, showing faint patchy ground glass infiltrates in lung bases, with significant improvement with respect to the previous study.
In subsequent check-ups, the patient reported a progressive decrease in bloody sputum until resolution. The chest CT performed 3 months after the previous study revealed a faint patchy ground glass pattern in the bases, with significant improvement with respect to the previous study (Fig. 1B). Respiratory function tests gave the following results: FEV1 2200ml (97.5%), FVC 3400ml (114%), FEV1/FVC 64.67%, DLCO 81.5%, and KCO 105.1%. Given this improvement, we decided to continue with apixaban, and the patient remained incident-free at the subsequent follow-up.
Around 0.5% of the population uses oral anticoagulants, of which the most widely used are vitamin K antagonists (VKA).2 The most significant complication related to the use of VKAs is bleeding, which is associated with an increase in mortality.2–4 Anticoagulant therapy is also a risk factor for the development of hemorrhages of respiratory origin even in patients without prior chronic respiratory illnesses, although the presence of the latter increases the risk.5 Additional risk factors include the following: history of chronic respiratory disease, advanced age, female sex, history of previous bleeding, uncontrolled arterial hypertension, kidney failure, liver disease, anemia, concomitant use of antiplatelet agents, and regular use of anti-inflammatory agents.2,4 An excessive antithrombotic effect (INR>3 or INR between 2 and 3 plus antiplatelet therapy) is known to favor bleeding. However, 20% of the major bleeds in an Italian series occurred in patients with INR<2, so it is important to take into account other factors that might increase the risk of bleeding.4
DOACs, such as oral Xa factor inhibitors (rivaroxaban, apixaban, and edoxaban) and an oral thrombin inhibitor (dabigatran), have been available since 2008. They were first used for the primary prevention of venous thromboembolic disease in adults undergoing elective hip or knee replacement surgery, and subsequently to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation, and in the secondary prophylaxis of venous thromboembolic disease. They are also indicated in other situations, for example rivaroxaban for secondary prevention in adult patients who have suffered an acute coronary syndrome with elevated biomarkers.2,3,6 DOACs have certain benefits over AVKs, such as a faster onset of action, no need for routine monitoring, a more predictable dose-response relationship, a shorter plasma half-life, a low risk of adverse effects, few interactions with drugs and food, improved efficiency, and the availability of specific antidote (idarucizumab) in the case of dabigatran. Other antidotes are in the process of approval, including andexanet for the reversal of factor Xa inhibitors.2,4,7 However, DOACs, like any anticoagulant treatment, always carry a risk of bleeding due to patient-associated factors, which may be increased in some situations, such as overdose, disease, or concomitant therapies that increase drug exposure, changes in hemostasis, or an urgent need for an invasive or surgical procedure.3,4 In clinical trials with DOACs, bleeding rates were generally low and comparable to those produced by low molecular weight heparin or warfarin in the case of dabigatran and rivaroxaban, or significantly lower in the case of apixaban (in the ARISTOTLE study, the rate of major bleeds was 2.13% per year in the apixaban group compared with 3.09% per year in the warfarin group, RR: 0.69; 95% CI: 0.60–0.80; P<0.001).3,8 In this regard, current cardiology guidelines recommend the preferential use of DOACs over AVKs in patients with non-valvular atrial fibrillation.9
In conclusion, anticoagulation is a predisposing factor for the development of bleeds, including those that originate in the lung. Overdosing, in the case of AVKs, is a major risk factor for this complication, but other factors that may increase the risk of bleeding must also be taken into account. DOACs have advantages with respect to AVKs, but as with any anticoagulant treatment there is always a risk of bleeding due to patient-associated factors.
Please cite this article as: Cerezo Lajas A, Rodríguez Guzmán MC, de Miguel Díez J. Hemoptisis en relación con el tratamiento con acenocumarol en rango terapéutico. Arch Bronconeumol. 2019;55:340–341.