Bronchial asthma is a complex disease in which genetic factors, environmental factors and oxidative damage are responsible for the initiation and modulation of disease progression. If antioxidant mechanisms fail, reactive oxygen species damage the biomolecules followed by progression of the disease. Catalase is one of the most important endogenous enzymatic antioxidants. In the present study, we examined the hypothesis that increased oxidative damage and polymorphism in the CAT gene (-262 promoter region, C/T) are associated with childhood bronchial asthma.
Patients and methodsGenotyping of the polymorphisms in the CAT gene in healthy (249) and asthmatic children (248) was performed using polymerase chain reaction – restriction fragment length polymorphism. Markers of oxidative damage: content of sulfhydryl groups and thiobarbituric acid-reactive substances were determined by spectrophotometry in children.
ResultsThe TT genotype of catalase was more frequent among the asthmatic patients (22.6%) than in healthy children (4.8%) (odds ratio=5.63; 95% confidence interval=2.93–10.81, P<.001). The amount of sulfhydryl groups decreased significantly and conversely, the content of thiobarbituric acid-reactive substances increased significantly in bronchial asthma and in catalase TT genotype compared to other catalase genotypes of this gene.
ConclusionsThese results suggest that catalase polymorphism might participate in development of bronchial asthma and in enhanced oxidative damage in asthmatic children. Genetic variation of enzymatic antioxidants may modulate disease risk.
El asma bronquial es una enfermedad compleja en la que los factores genéticos, los factores ambientales y la lesión oxidativa son responsables del inicio y la modulación de su progresión. Si fracasan los mecanismos antioxidantes, las especies reactivas del oxígeno afectan a las biomoléculas, lo que se sigue de la progresión de la enfermedad. La catalasa es uno de los antioxidantes enzimáticos endógenos más importantes. En el presente estudio examinamos la hipótesis de que un aumento de la lesión oxidativa y el polimorfismo en el gen CAT (región promotora -262 C/T) se asocian a asma bronquial infantil.
Pacientes y métodosEn niños sanos (249) y niños asmáticos (248) se efectuó una genotipificación de los polimorfismos en el gen CAT usando la reacción en cadena de la polimerasa-polimorfismo de longitud de fragmentos de restricción. Mediante espectrofotometría, en los niños se analizaron los marcadores de lesión oxidativa: el contenido de grupos sulfhidrilo y de sustancias reactivas al ácido tiobarbitúrico.
ResultadosEl genotipo TT de la catalasa fue más frecuente entre pacientes asmáticos (22,6%) que en niños sanos (4,8%) (odds ratio=5,63; intervalo de confianza del 95%=2,93–10,81; p<0,001). El contenido de grupos sulfhidrilo disminuyó significativamente y, al contrario, el contenido de sustancias reactivas a ácido tiobarbitúrico aumentó significativamente en el asma bronquial y el genotipo TT de catalasa comparado con los otros genotipos catalasa de este gen.
ConclusionesLos resultados del presente estudio sugieren que el polimorfismo del gen de la catalasa podría participar en la aparición de asma bronquial y en el aumento de la lesión oxidativa en niños asmáticos. La variación genética de los antioxidantes enzimáticos podría modular el riesgo de la enfermedad.
Bronchial asthma is a chronic, complex, heterogeneous disease of the airways, which includes the activation of many inflammatory and structural cell populations that release numerous inflammatory mediators, giving rise to the physiopathological changes that are characteristic of the disease.1 Environmental and genetic factors play a role in its onset, although the exact mechanisms of their actions have not been fully determined. The lungs are continually exposed to oxidants generated endogenously from the mitochondria, phagocytes and other cells, or exogenously from air pollutants and cigarette smoke. They have the largest endothelial surface area of any organ, which makes the lung tissue the principal target for circulating oxidants and xenobiotics. Asthmatic patients produce a variety of mediators, including reactive oxygen species (ROS).2 An increase in oxidative damage can contribute to both the origin and development of respiratory diseases, including bronchial asthma. Lung lesions due to ROS are related with the oxidation of deoxyribonucleic acid (DNA), proteins and lipids. These oxidized biomolecules can induce various responses and a cascade of events, such as airway hyperreactivity, increase in the generation of chemoattractants, release of tachykinins and neurokinins, and an increase in the release of mediators which ultimately aggravate the oxidative damage.1 The lungs can use non-enzymatic antioxidants, such as vitamins, uric acid, glutathione, sulfhydryl groups (proteins, peptides and amino acids) and various enzymatic antioxidants, such as superoxide dismutase, catalase (CAT) and glutathione peroxidase as a defense against oxidative damage and ROS.
CAT (EC 1.11.1.6) is a common antioxidant enzyme responsible for controlling the hydrogen peroxide concentrations in cells. It is ubiquitous in most aerobic cells also detected in the lungs (macrophages, fibroblasts and pneumocytes).3 As an intracellular antioxidant enzyme, CAT catalyses the breakdown of two hydrogen peroxide molecules into one oxygen molecule and two water molecules; its activity is determined genetically. The CAT gene is located on chromosome 11p13; it is 34kb long, contains 13 exons and 12 introns and codes a 256-amino acid protein.4,5 Various polymorphisms of this enzyme have been described and characterized, both in the coding6,7 and non-coding region.7–11 A common polymorphism in the CAT gene promoter region is the substitution of T for C in position T -262 in the 5′ region,9 which is considered to lead to a decrease in enzyme activity. The CAT TT genotype could be responsible for a reduction in the antioxidant defense and a subsequent increase in oxidative damage.
Since oxidative damage plays a role in the pathogenesis of asthma, and CAT is critical for protecting cells against ROS, we hypothesized that the polymorphisms in the CAT gene that influence its enzyme activity contribute substantially to the onset of the disease. This study aimed to evaluate CAT polymorphism -262 C/T in asthmatic children. We sought to determine markers of oxidative damage, and to analyze a possible association between the CAT genotype and oxidative damage of proteins and lipids in asthmatic and healthy children.
Patients and MethodsStudy IndividualsThe study population consisted of 457 children. Using the categories from reference questionnaires, the asthma history of the children was recorded: age, sex, exposure to cigarette smoke and family history of asthma, wheezing and allergy. The children or their parents completed the Asthma control test®. Active smokers and children exposed to passive smoking were then excluded from the study. The 248 asthma patients (58% boys and 42% girls) were enrolled from the Pediatrics Department of Martin University Hospital (Slovakia). During routine health checks, general practitioners recruited a further 249 healthy children of comparable age and sex (54% boys and 46% girls). The children did not present any clinical symptoms of allergic diseases and had no history of serious illness. Asthmatic children who participated in the study were characterized by recurrent airway obstruction, manifested by wheezing and dyspnea, with spontaneous relief with bronchodilator treatment (as defined in the Global Asthma Initiative).
All children underwent fractional expired nitric oxide (FENO) and expired carbon monoxide (eCO) analyses. The FENO was determined according to American Thoracic Society/European Respiratory Society (ATS/ERS) standards12 using a portable nitric oxide analyzer (NIOX-MINO®, Aerocrine, Sweden). The eCO was analyzed using a Micro 4 Smokerlyzer® (Bedfont, England). We also carried out basic spirometry (KoKo DigiDoser-Spirometer, nSpire Health, Louisville, United States), in accordance with ATS/ERS recommendations.13 The FENO and eCO values were estimated before the spirometer test.
The study was approved by the Jessenius Faculty of Medicine, Martin, and written informed consent was obtained from the parents of all the children examined.
Isolation of Deoxyribonucleic AcidBlood samples were extracted into ethylene-diamine-tetraacetic acid (EDTA) tubes. The genomic DNA was prepared using blood according to a commercial procedure (Wizard® Genomic DNA purification kit, Promega, Madison, USA).
Determination of Catalase GenotypesAll DNA samples from asthmatic and healthy children were genotyped by polymerase chain reaction (PCR), followed by restriction fragment length polymorphism (RFLP) analysis. CAT polymorphism -262 C/T (rs 1001179) was determined using direct primer 5′-AGAGCCTCGCCCCGCCGGACCG-3′ and CAT reverse primer 5′-TAAGAGCAGAGAAAGCATAGCT-3′. PCR products of 185bp were digested using the restriction enzyme Smal. The products were visualized by 2% agarose gel electrophoresis. The wild-type CC genotype appeared as 155 and 30bp fragments, and the CT genotype as 185, 155 and 30bp fragments. TT genotypes were not digested by Smal (185bp).
Plasma Protein Sulfhydryl GroupsPlasma for determination of protein and lipid markers of oxidative damage was obtained from blood prepared by centrifugation for 20min, at 2000×g at 4°C.
The total concentration of reduced sulfhydryl groups (−SH, proteins, oligopeptides, glutathione and amino acids) was determined by spectrophotometry.14 Two ml of Ellman's reagent (0.250mol/l of Tris–HCl; pH 8.2, 10mmol/l of 5.5′-dithiobis-2-nitrobenzoic acid) were added to 100μl of sample and incubated for 15min at room temperature. The absorbance was read at 412nm and –SH group content was calculated using the molar absorption coefficient of 13600M−1cm−1 after subtracting the absorbance of the blank from the sample absorbance. A population of 249 healthy individuals was used to determine the normal value.
Lipid Peroxide AnalysisModifications in the structure of the plasma lipids were analyzed by determining the thiobarbituric acid-reactive substances (TBARS).15 A sample was incubated with ethanol, 14% trichloroacetic acid and 0.6% thiobarbituric acid at 80°C for 30min. It was then incubated for 5min at 0°C and centrifuged for 10min at 2000×g at 25°C. The TBARS concentration was determined from reading the absorbance at 532nm. The population of 249 healthy individuals was used to determine the normal value.
Statistical AnalysisThe results from both groups of individuals were compared using ANOVA, the Student's t-test and Chi-squared test (χ2). The association of CAT gene polymorphism -262 C/T with bronchial asthma was determined using the Pearson χ2 test or Fisher's exact test. The genotype distribution was examined for deviation from the Hardy-Weinberg equilibrium. To analyze the frequencies of the CAT genotypes in patients with bronchial asthma, compared with healthy children, the odds ratio (OR) and confidence intervals (95% CI) were used. Pearson's correlation was used to calculate the relationship between the oxidative stress markers and inflammatory markers in the expired air (with Spearman's correlation when indicated). All values are presented as mean±SEM. A P value <.05 was considered statistically significant.
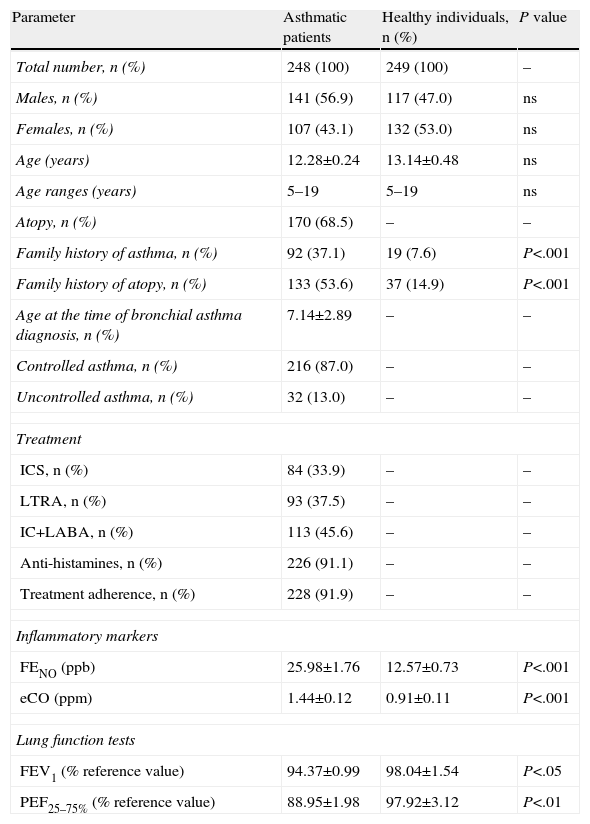
ResultsCatalase Gene Polymorphism in Children With Bronchial Asthma and Healthy IndividualsTable 1 shows the demographic and other characteristics of the study population. A positive family history of asthma was detected in 92 asthmatic children (37.1%) and in 19 healthy children (7.6%). Based on a positive skin prick test with a standard panel of inhaled allergens, 170 patients (68.5%) were atopic and 78 (31.5%) presented a non-atopic variant of asthma.
General Characteristics of the Study Population.
| Parameter | Asthmatic patients | Healthy individuals, n (%) | P value |
| Total number, n (%) | 248 (100) | 249 (100) | – |
| Males, n (%) | 141 (56.9) | 117 (47.0) | ns |
| Females, n (%) | 107 (43.1) | 132 (53.0) | ns |
| Age (years) | 12.28±0.24 | 13.14±0.48 | ns |
| Age ranges (years) | 5–19 | 5–19 | ns |
| Atopy, n (%) | 170 (68.5) | – | – |
| Family history of asthma, n (%) | 92 (37.1) | 19 (7.6) | P<.001 |
| Family history of atopy, n (%) | 133 (53.6) | 37 (14.9) | P<.001 |
| Age at the time of bronchial asthma diagnosis, n (%) | 7.14±2.89 | – | – |
| Controlled asthma, n (%) | 216 (87.0) | – | – |
| Uncontrolled asthma, n (%) | 32 (13.0) | – | – |
| Treatment | |||
| ICS, n (%) | 84 (33.9) | – | – |
| LTRA, n (%) | 93 (37.5) | – | – |
| IC+LABA, n (%) | 113 (45.6) | – | – |
| Anti-histamines, n (%) | 226 (91.1) | – | – |
| Treatment adherence, n (%) | 228 (91.9) | – | – |
| Inflammatory markers | |||
| FENO (ppb) | 25.98±1.76 | 12.57±0.73 | P<.001 |
| eCO (ppm) | 1.44±0.12 | 0.91±0.11 | P<.001 |
| Lung function tests | |||
| FEV1 (% reference value) | 94.37±0.99 | 98.04±1.54 | P<.05 |
| PEF25–75% (% reference value) | 88.95±1.98 | 97.92±3.12 | P<.01 |
eCO, expired carbon monoxide; FENO, fractional expired nitric oxide; FEV1, forced expiratory volume in the first second; ICS, inhaled corticosteroids; LABA, long-acting beta2 agonists; LTRA, leukotriene receptor agonist; n, number of individuals; ns, not significant; PEF, peak expiratory flow; ppb, parts per billion; ppm, parts per million.
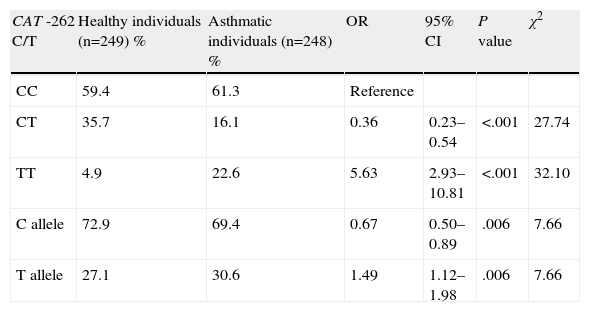
The genotype distribution and allele frequency in asthmatic and healthy children are shown in Table 2. A statistically significant difference was identified in the distribution of genotype polymorphisms of the CAT gene between asthmatic and healthy children. In patients with bronchial asthma, there was a higher prevalence of CAT genotype TT (22.6%) than in control individuals (4.8%) (Table 2). There was a 5.63-fold increase in the risk of asthma in the CAT TT genotype (OR=5.63; 95% CI=2.93–10.81; P<.001). The coefficient of inbreeding (consanguinity) in control individuals was −0.019 and 0.610 in the cases. The C allele was the most common in asthmatics (72.9%) and in healthy individuals (69.4%). The T allele represented a potential risk factor (OR=1.49; 95% CI=1.12–1.19; P<.01). The combination of the CC+CT genotypes was shown to be a positive factor (OR=0.178; 95% CI=0.65–1.33, P<.001, χ2=32.10). The combination of the heterozygous variant with the homozygous variant did not show any significant association.
Distribution of Genotypes and Alleles of the Catalase Gene (CAT) and Risk of Onset of Bronchial Asthma.
| CAT -262 C/T | Healthy individuals (n=249) % | Asthmatic individuals (n=248) % | OR | 95% CI | P value | χ2 |
| CC | 59.4 | 61.3 | Reference | |||
| CT | 35.7 | 16.1 | 0.36 | 0.23–0.54 | <.001 | 27.74 |
| TT | 4.9 | 22.6 | 5.63 | 2.93–10.81 | <.001 | 32.10 |
| C allele | 72.9 | 69.4 | 0.67 | 0.50–0.89 | .006 | 7.66 |
| T allele | 27.1 | 30.6 | 1.49 | 1.12–1.98 | .006 | 7.66 |
CI, confidence interval; n, number of individuals; OR, odds ratio.
The results of both groups of individuals were compared using ANOVA, the Student's t-test and Chi-squared test (χ2). The association of CAT gene polymorphism -262 C/T with bronchial asthma was determined using the Pearson χ2 test or Fisher's exact test.
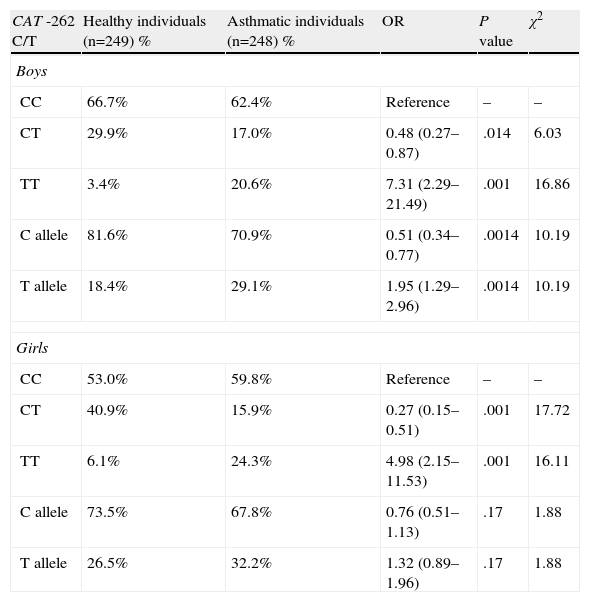
We separated the study population according to sex (Table 3). No significant difference was found in the distribution of the CAT genotypes and alleles between asthmatic boys and girls. A statistically significant difference was found in the distribution of the CAT genotypes and alleles when asthmatic boys or girls were compared with healthy boys or girls. The homozygous variant was associated with bronchial asthma in both sexes (OR=7.31; 95% CI=2.29–21.49; respectively, OR=4.98; 95% CI=2.15–11.53; P<.001).
Distribution of Genotypes and Alleles of the Catalase Gene (CAT) and Risk of Onset of Bronchial Asthma in Boys and Girls.
| CAT -262 C/T | Healthy individuals (n=249) % | Asthmatic individuals (n=248) % | OR | P value | χ2 |
| Boys | |||||
| CC | 66.7% | 62.4% | Reference | – | – |
| CT | 29.9% | 17.0% | 0.48 (0.27–0.87) | .014 | 6.03 |
| TT | 3.4% | 20.6% | 7.31 (2.29–21.49) | .001 | 16.86 |
| C allele | 81.6% | 70.9% | 0.51 (0.34–0.77) | .0014 | 10.19 |
| T allele | 18.4% | 29.1% | 1.95 (1.29–2.96) | .0014 | 10.19 |
| Girls | |||||
| CC | 53.0% | 59.8% | Reference | – | – |
| CT | 40.9% | 15.9% | 0.27 (0.15–0.51) | .001 | 17.72 |
| TT | 6.1% | 24.3% | 4.98 (2.15–11.53) | .001 | 16.11 |
| C allele | 73.5% | 67.8% | 0.76 (0.51–1.13) | .17 | 1.88 |
| T allele | 26.5% | 32.2% | 1.32 (0.89–1.96) | .17 | 1.88 |
CI, confidence interval; n, number of individuals; OR, odds ratio.
The results of both groups of individuals were compared using ANOVA, the Student's t-test and Chi-squared test (χ2). The association of CAT gene polymorphism -262 C/T with bronchial asthma was determined using the Pearson χ2 test or Fisher's exact test.
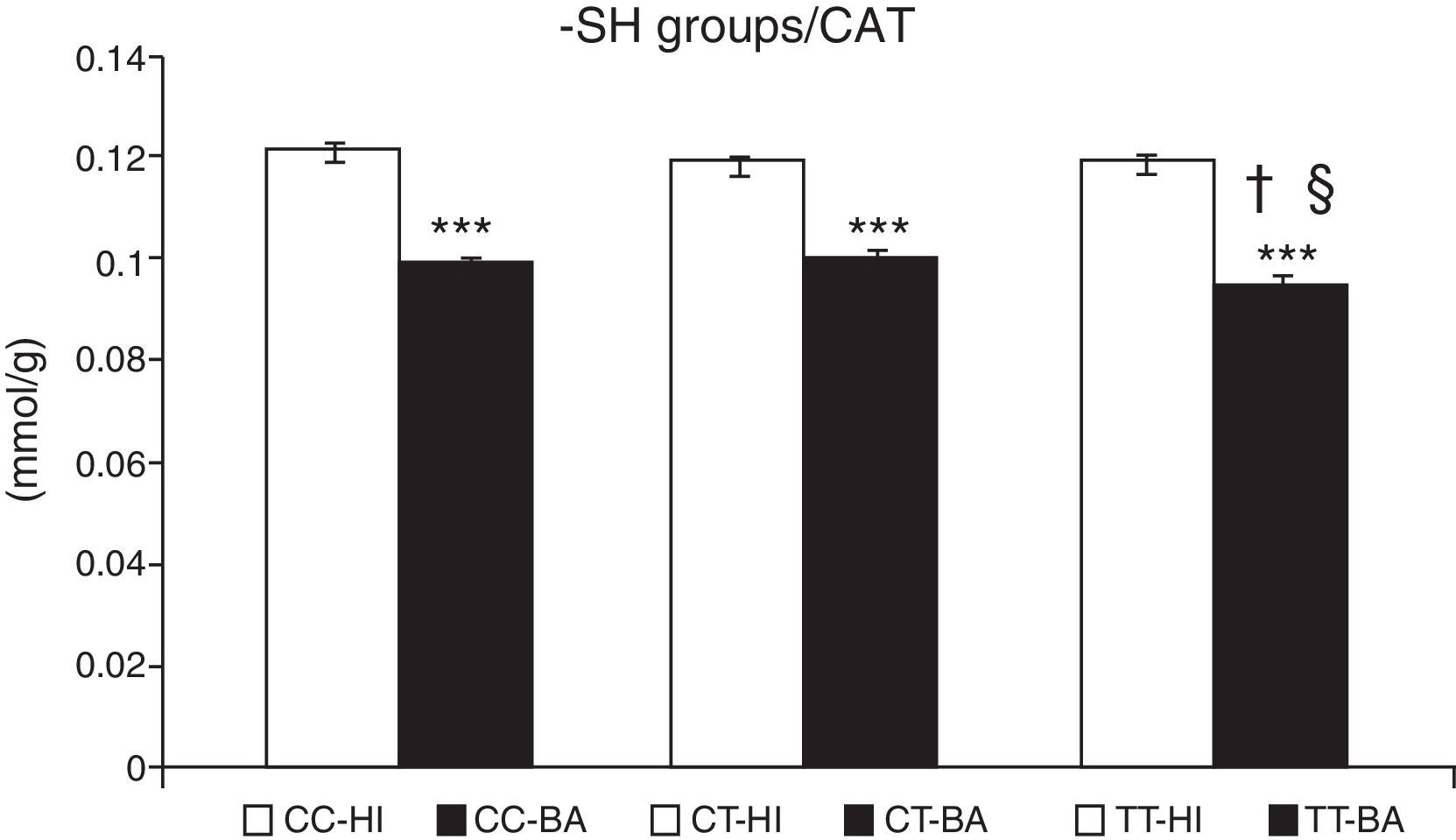
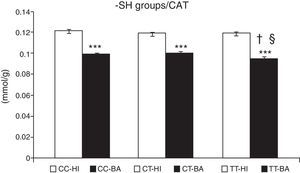
Greater oxidative damage of proteins and lipids was detected in children with bronchial asthma compared with healthy children (Table 4). The concentration of –SH groups decreased in the order of 18.8%±0.7% (P<.001) in asthmatic children, compared to control individuals. There was no significant difference between the –SH group content between children with asthma exacerbations and controlled asthma. No differences were observed in the control individuals in the concentration of –SH groups according to the CAT genotype (Fig. 1). A lower concentration of –SH groups was evident in patients with the CAT TT genotype (P<.05), compared to those with the CC and CT genotype (P<.05) (Fig. 1).
Markers of Oxidative Damage of Proteins and Lipids.
| Individuals | –SH, mmol/g | TBARS, nmol/mg |
| Healthy | 0.1216±0.0019 | 0.0623±0.002 |
| Asthmatics | 0.0623±0.002*** | 0.0809±0.002*** |
–SH: sulfhydryl group; TBARS: thiobarbituric acid reactive substances.
The results of both groups of individuals were compared using ANOVA and the Student's t-test. The results are expressed as mean±standard error of the mean.
Concentration of sulfhydryl groups (–SH). Comparison of the concentration of –SH in healthy individuals (HI) and children with bronchial asthma (BA) according to the catalase genotype. ***P<.001, comparison of the catalase genotypes, †P<.05, comparison of the catalase TT genotype versus the CC genotype in patients, §P<.05, comparison of the catalase TT genotype versus the CT genotype in patients.
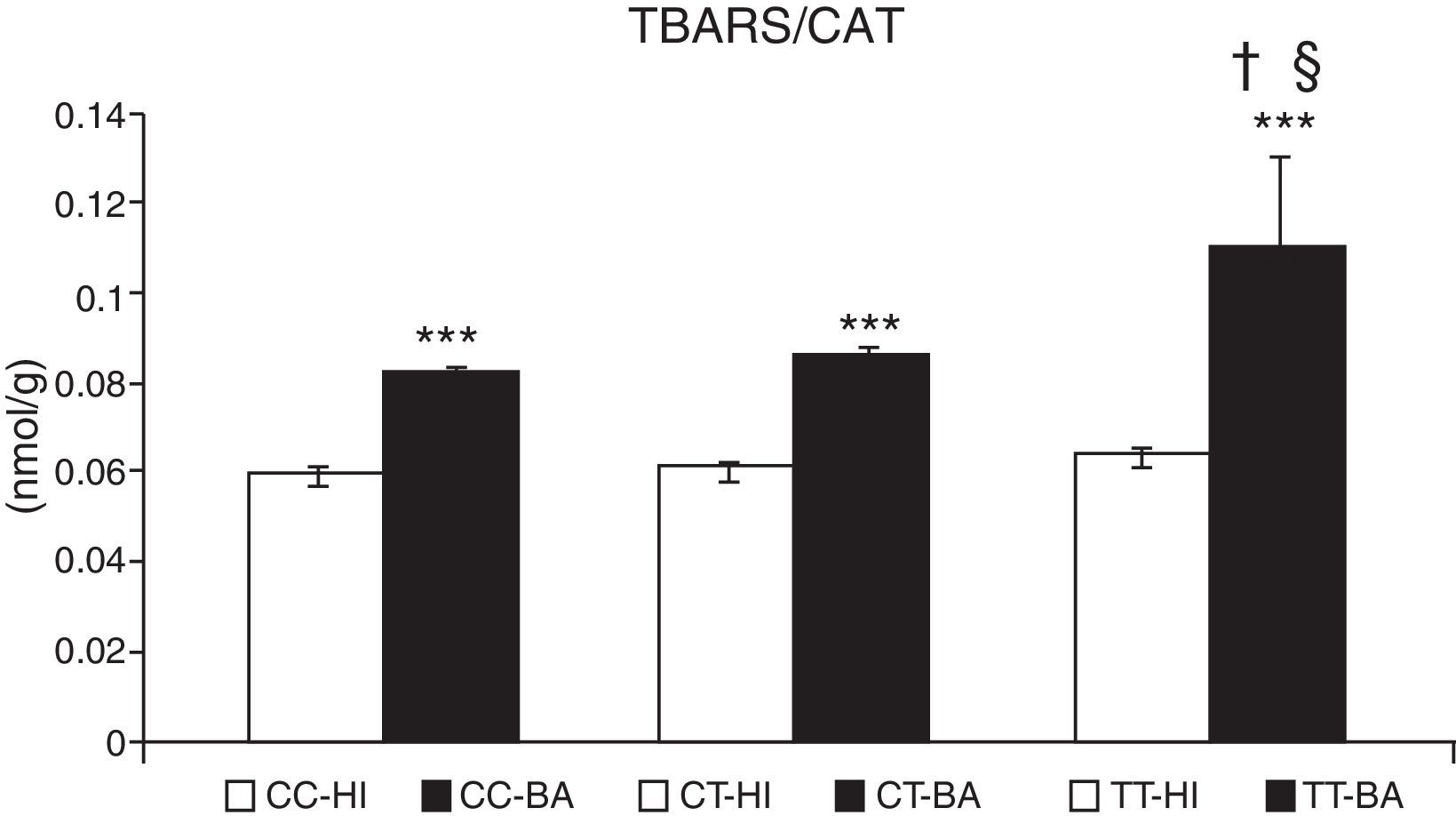
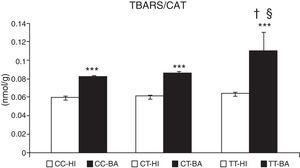
Oxidative stress caused an accumulation of TBARS in children with bronchial asthma. The concentration of these substances increased by 29.9%±3.2% (P<.001) in patients. In patients with asthma exacerbation, the lipid peroxidation markers increased significantly by 33.0%±3.0%, compared to patients whose asthma was stable (P<.05). The TBARS values did not change according to the CAT genotype in healthy individuals (Fig. 2). In asthmatics with CAT genotype TT, a higher concentration of TBARS was identified compared with the CC genotype (P<.05), and also with the CT genotype (P<.05) (Fig. 2).
Concentration of thiobarbituric acid-reactive substances (TBARS). Comparison of the concentration of TBARS in healthy individuals (HI) and children with bronchial asthma (BA) according to the catalase genotype. ***P<.001, comparison of the catalase genotypes, †P<.05, comparison of the catalase TT genotype versus the CC genotype in patients, §P<.05, comparison of the catalase TT genotype versus the CT genotype in patients.
We found a significant correlation between markers of oxidative damage and inflammatory parameters in the expired air of asthmatic patients. There was a slight significantly positive correlation between the TBARS concentration and the expired air markers (FENO versus TBARS: r=0.232 [P=.002]; eCO versus TBARS: r=0.147 [P=.044]). A slight or moderately significant negative correlation was observed between the concentration of –SH groups and inflammatory markers (FENO versus –SH: r=−0.343 [P<.001]; eCO versus –SH: r=−0.232 [P=.001]). These correlations were not observed in the control group.
DiscussionBronchial asthma is a complex immunological disease, affected by environmental and genetic factors and their interactions, which are probably key in its pathogenesis and prognosis. Asthma belongs to a group of disorders in which oxidative damage and an imbalance between pro-oxidant and antioxidant substances play an important role. We observed a higher prevalence of the TT genotype of the antioxidant enzyme CAT and an increase in the oxidative damage of proteins and lipids associated with bronchial asthma. We also found that in asthmatic children with the CAT TT genotype, there was greater oxidative damage compared with the other genetic variation of CAT -262 C/T. As far as the authors are aware, this is the first study on the association of genetic polymorphism and oxidative damage in Slovakian children or adults with asthma.
Polymorphism of the genes involved in oxidative stress pathways, NAD(P)H:quinone oxidoreductase16 and glutathione transferases M1 and P1,17,18 have been associated with bronchial asthma.16,17 CAT is one of the essential antioxidant enzymes and, therefore, CAT is a candidate gene for many diseases that are potentially related with oxidative damage and exogenous/endogenous oxidative stress. In this study, the frequency of the CAT TT genotype was 0.226 in asthmatic children and 0.048 in healthy children (P<.001). The frequency of CAT genotype -262 C/T was comparable to that described in other European Studies (English, German, Polish and Turkish).18–21 Analysis according to sex showed similar results to the analysis of the total population, and the homozygous variant was associated with bronchial asthma. We have demonstrated that polymorphism in the CAT gene may be associated with a predisposition to bronchial asthma. The homozygous variant CAT -262 C/T was associated with asthma in white children of Hispanic origin but not in children of other origins.22 Polonikov et al. (2009) did not observe differences in the frequencies or genotype of CAT gene polymorphism -262 C/T between asthmatic adults and healthy controls.23 However, an association was observed between CAT gene polymorphism -21A/T and bronchial asthma, and it was found that the risk of asthma in carriers of the -21AA genotype depends on exposure to both oxidants and antioxidants. The contradictory results with respect to the relationship between the CAT gene polymorphism and bronchial asthma may be explained by the influence of the ethnicity. In our selected population of Slovakian children, the TT genotype was associated with bronchial asthma. This genetic variation of the CAT gene could be responsible for oxidative damage, as we observed an increase particularly in asthmatic children who were carriers of the TT genotype. A decrease has been observed in CAT activity in the TT genotype in various studies.11,24,25 However, Forsberg et al.9 found a higher level of CAT in the CAT gene TT genotype. The higher risk of asthma could be a result of the decreased CAT activity in the TT genotype, and the subsequent increase in the oxidative damage of the biomolecules. We observed an increase in protein and lipid oxidative damage in children with bronchial asthma. Chronic inflammation is associated with higher production of ROS and an increase in oxidative stress in the lung. Higher levels of hydrogen peroxide, superoxide radical27 and lipid peroxidation27,28 have been found in children with bronchial asthma. In the asthmatic population in the present study, we observed an increase in nitric oxide29 and eCO. These markers are characterized by a higher concentration in the TT genotype of asthmatic children compared with the CC and CT genotypes (results not published). It has been shown that the combination of the heterozygous variant with the homozygous variant is positive. A C allele could be sufficient for CAT to function, but further genetic studies are required to determine the activity and function of CAT in different genotypes, not only for this polymorphism. In healthy children with a variant of the CAT genotype, other enzymatic antioxidants (such as heme oxygenase, superoxide dismutase, glutathione peroxidase and glutathione transferase) are detected in the normal homozygous form, which protect them against oxidative damage. Moreover, healthy children do not show the increased ROS values detected in asthmatic children. The relationship between oxidative damage and chronic inflammation in asthmatics is probably two-way. Oxidative stress can exacerbate existing inflammation and can contribute to airway remodeling and conversely, continuous chronic inflammation may result in increased production of ROS and greater oxidative damage. In the control group, comparison of the concentration of oxidative damage markers according to the CAT genotype did not reveal significant differences. In this study, the higher lipid peroxidation and protein modification detected could be a result of excessive ROS production or reduced capacity of the antioxidant defense system in asthmatics. The antioxidants in food help endogenous antioxidants to prevent oxidative damage and represent a possible therapeutic option. Antioxidant treatment could enhance reference asthma treatment and be an adjuvant, especially in asthmatic patients with antioxidant enzyme risk genotypes. Interactions between genes and environmental factors may influence CAT activity in the different genotypes. We did not analyze these effects, but it has been shown that exposure to smoke and the consumption of fruit and vegetables affect the activity of CAT in the CC, CT and TT genotypes of the CAT gene.24–26,28
Bronchial asthma is a complex, multifactor disease in which genetic factors, environmental factors and oxidative damage are responsible for its onset, modulation and progression. The results of this study show that polymorphisms of the CAT gene may be associated with bronchial asthma in children, and could participate in greater oxidative damage. The genetic variation in CAT, which protects the cells against ROS, may affect the asthmatic process. In summary, asthma is not an individual disease, but a group of diseases associated with an increase in oxidative stress, followed by the accumulation of oxidative damage. This could be an important factor that contributes to the development and persistence of airway inflammation in asthmatic children. Greater oxidative damage may be a consequence of cross reactions between polymorphism of the CAT gene and environmental factors, as well as polymorphism of the CAT gene and other genetic factors that may modify the risk of disease. However, further studies on the interactions between polymorphism of the CAT gene and additional polymorphisms in genes related with asthma are required, as they could help to improve our understanding of the complex disease that is bronchial asthma.
FundingThis study was funded with a grant from the Ministry of Health 2007/47-UK-12, and by the Ministry of Education, Science, Research and Sports of the Slovak Republic, VEGA 1/0071/11.
Conflict of InterestNone of the authors have declared any conflict of interests.
We would like to thank J. Bencatova, Z. Cetlova and A. Kempna for their laboratory assistance.
Please cite this article as: Babusikova E, Jesenak M, Evinova A, Banovcin P, Dobrota D. Frecuencia del polimorfismo -262 C/T en el gen de la catalasa y lesión oxidativa en niños eslovacos con asma bronquial. Arch Bronconeumol. 2013;49:507–512.