Common variable immunodeficiency is one of the most frequent immunity alterations. The most common clinical presentation occurs with recurrent respiratory infections, from pneumonia to otitis, and may be associated with other diseases such as bronchiectasis or interstitial lung diseases. We report the case of a 28-year-old patient with frequent respiratory infections and nodular pulmonary infiltrates, who was diagnosed with common variable immunodeficiency and follicular bronchiolitis. In some of the cases, follicular bronchiolitis is associated with immunodeficiencies and should be included in a differential diagnosis with lymphoid nodular hyperplasia, lymphocytic interstitial pneumonia and low-grade BALT lymphoma.
La inmunodeficiencia común variable es una de las alteraciones de la inmunidad más frecuentes. Suele manifestarse con infecciones respiratorias de repetición, desde neumonías hasta otitis y puede asociarse a otras patologías como bronquiectasias o enfermedades intersticiales. Presentamos el caso de un paciente de 28 años con infecciones respiratorias frecuentes e infiltrados nodulares pulmonares, que fue diagnosticado de Inmunodeficiencia Común Varible y bronquiolitis folicular. La bronquiolitis folicular se asocia, en algunos casos, a inmunodeficiencias y debe realizarse un diagnóstico diferencial con la hiperplasia nodular linfoide, neumonía intersticial linfocítica y linfoma BALT de bajo grado.
Common variable immunodeficiency (CVID) is one of the most well-known primary immunodeficiencies, with an incidence of 1/10000 patients in the general population.1 Symptoms appear in children as well as in adults and frequently include repeated respiratory infections. CVID may be associated with other pulmonary pathologies, such as interstitial disease.
Case ReportThe patient is a 28-year-old male who works in the hotel industry, with no toxic habits of any kind. He has an ocular prosthesis due to congenital microphthalmia in the left eye. He came to our pulmonology department due to respiratory symptoms that had been affecting him for the previous four years. He reported repeatedly having colds and respiratory infections, which were treated with multiple cycles of antibiotics but showed no clear improvement in his symptoms, that included cough and persistent greenish expectoration. Upon examination, the patient presented with pale skin and mucosae along with reduced vesicular murmur in both lung bases and crackles in the left lung base.
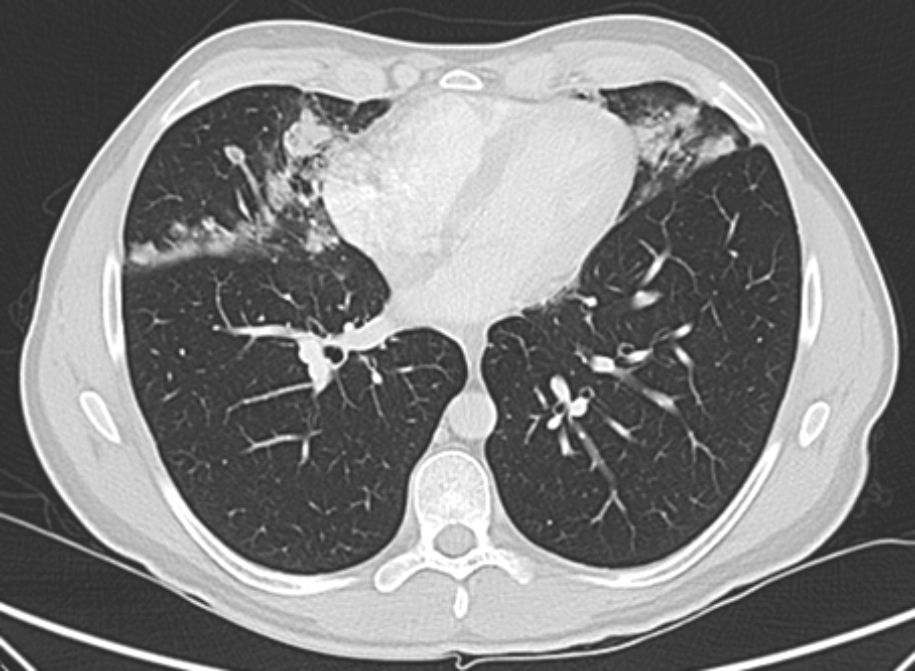
Posteroanterior and lateral chest radiographs and thoracic computed tomography (CT) showed the presence of irregular nodules (acinar) and alveolar infiltrates in the middle lobe, lingula and lower lobes, with perilymphatic and subpleural distribution (Fig. 1). Pulmonary function tests were normal, with FVC 4830ml (97%), FEV1 3870ml (93%), FEV1/FVC 80%, DLCO 94%, KCO 94% and VA 97%. We ordered a thorough complete blood analysis and bronchoscopy with transbronchial biopsy by cryocatheter.
Blood analysis demonstrated low platelet count (84000×103μl) and reduced immunoglobulin levels, with IgG <140mg/dl (700–1600), IgA <33mg/dl (70–400), IgM <21mg/dl (40–230), IgE 1.8mg/dl (23–94), and low IgG subclasses: subclass 1, 57mg/dl (402–712): subclass 2, 4mg/dl (216–523); subclass 3, 53mg/dl (36–139); subclass 4, <1mg/dl (9–104).
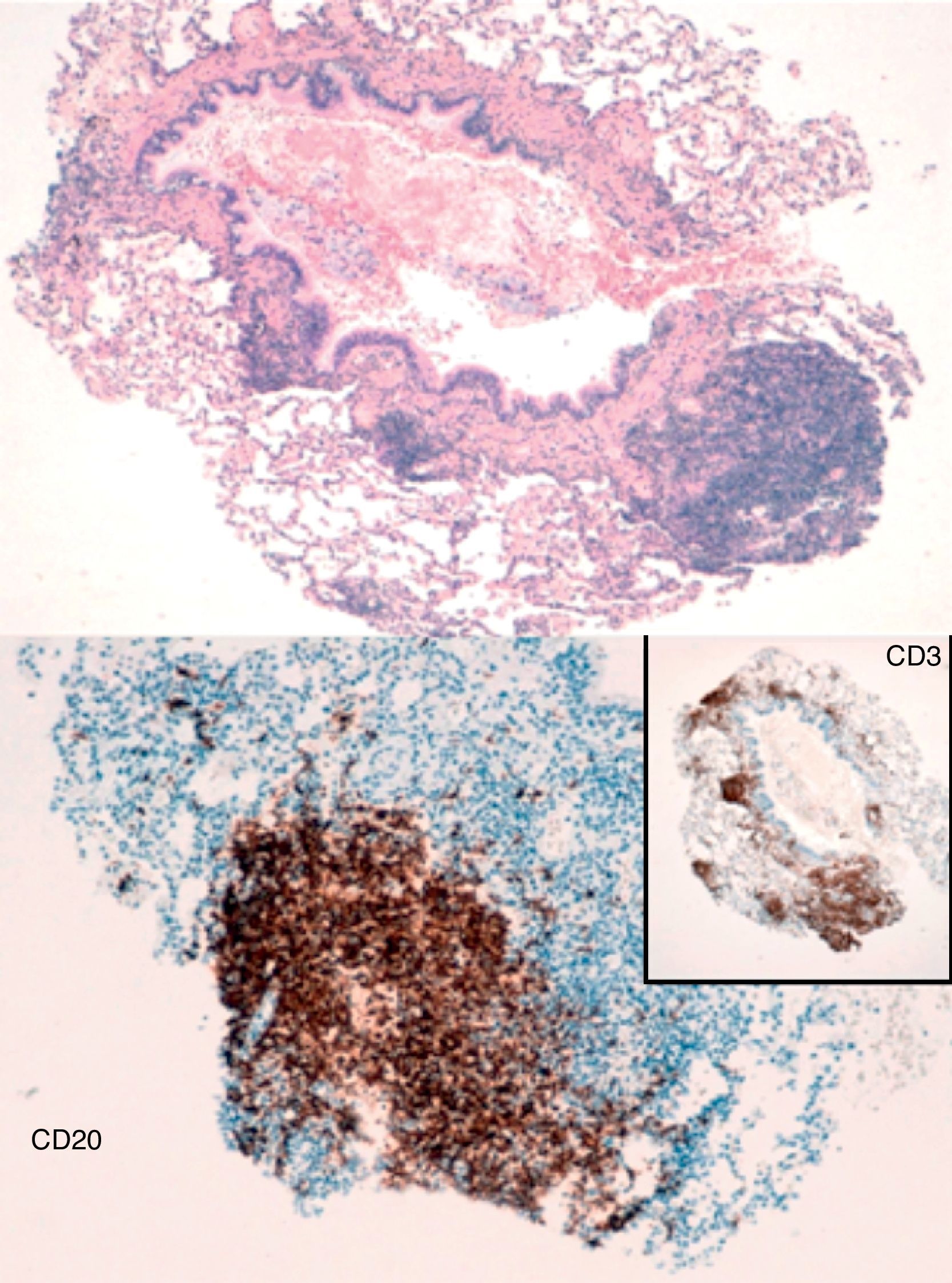
The biopsy results reported fragments of lung parenchyma with interstitial lymphoid aggregates that were predominantly adjacent to bronchioles and lymphoid follicles. Immunohistochemistry detected a predominant T-cell component and prevalence of CD4+ population with limited reactive B-cell aggregates. All this was compatible with reactive lymphoid hyperplasia with images of follicular bronchiolitis (Fig. 2). The patient was sent to hematology, where he was diagnosed with CVID and treatment was started with intravenous gamma globulin.
DiscussionCVID is a primary immunodeficiency with absence of antibodies that is associated with multiple clinical phenotypes. Its reported incidence is variable: from 1/25000 to 1/50000 newborns, depending on the series.2 Two peaks have been identified for the onset of the disease: from 1 to 5 and from 16 to 20 years of age.
There are multiple respiratory manifestations, which frequently entail repeated infections like pneumonia, otitis media or sinusitis, usually caused by encapsulated pathogens. Other respiratory manifestations are bronchiectasis or interstitial diseases: granulomatous diseases, lymphocytic interstitial pneumonia, non-specific interstitial pneumonia or organizing pneumonia.1
Recently, the term granulomatous-lymphocytic interstitial lung disease (GLILD) has been used to describe when a patient with CVID presents with coexisting histologic data for granulomatous disease and lymphoproliferative disease (lymphocytic interstitial pneumonia, follicular bronchiolitis, lymphoid hyperplasia).3 Frequently, there is the presence of mediastinal, hilar, axillary and abdominal lymphadenopathies as well as hepatosplenomegaly. The prognosis of patients, who present this entity is reduced by half, being about 30 years in those without associated GLILD.4
Radiologically, it is characterized by the existence of areas of consolidation, ground-glass nodules and thickened interlobular septa predominantly in the lower lobes.3
In our case, the anatomic pathology findings were compatible with follicular bronchiolitis. This entity is characterized by hyperplasia of lymphoid tissue throughout the airway and by the development of lymphoid follicles and germinal centers with peribronchial or peribronchiolar distribution. This may be the only finding or it may be associated with bronchiectasis, asthma, etc. There is usually underlying collagen disease, rheumatoid arthritis or immunodeficiency, which can be either congenital, acquired (like AIDS and CVID), or idiopathic.5
It has been described at any age, although when the disease is not associated with collagen disease or immunodeficiency it tends to appear in middle-aged individuals. Symptoms are non-specific, and from a respiratory function standpoint, any pattern may be seen and can even be normal.6
Follicular bronchiolitis could be considered a manifestation of nodular lymphoid hyperplasia as an immune response against an antigen, with the consequent lymphocytic proliferation. Nevertheless, most patients do not develop follicular bronchiolitis after antigenic exposure; therefore, this stimulation would not be sufficient to explain the pathogenesis. Two stages can be defined in its development after the onset of symptoms, with an initial exposure to antigens that stimulate the BALT system, and then the immunologic dysfunction leads to lymphocytic proliferation.7
The most common radiologic findings are bilateral nodular opacities, generally smaller than 3mm, although cases with opacities larger than 12mm have been described, with centrilobular or peribronchiolar distribution. Bilateral patchy areas of ground glass opacities can be seen with non-segmental distribution.8
From a histologic standpoint, there are numerous lymphoid follicles associated with germinal centers around the bronchi and bronchioles, which are occasionally associated with interstitial inflammatory infiltrates in the adjacent alveolar septa. In 20% of the cases, this is significant and the differential diagnosis should include lymphocytic interstitial pneumonia, lymphoid nodular hyperplasia and low-grade BALT lymphoma.5
Differentiating between these entities is not simple and is usually based on the extension of the infiltrate, with a peribronchial or peribronchiolar distribution in follicular bronchiolitis and diffuse distribution in lymphocytic interstitial pneumonia.8
Lymphoid nodular hyperplasia is distinguished by its radiological as well as histologic characteristics. It presents as single or multiple masses with proliferation of germinal centers that tend to converge, with interfollicular plasma cells. In low-grade BALT lymphoma, these masses appear to be solid and well-defined or multiple, with infiltrates made up of monoclonal B lymphocytes, and in some of the cases there may be germinal centers.5
Even though treatment with immunoglobulins can significantly reduce the frequency and severity of infections, the lung lesions progress in spite of proper treatment. The number of pneumonias prior to diagnosis has been proposed as the most sensitive parameter for detecting alterations on thoracic CT.9
In these patients, diagnosis is frequently delayed due to the low prevalence and low clinical suspicion, so the presence of repeated pneumonia is sufficient criteria to contemplate the existence of an immunodeficiency.
Please cite this article as: Camarasa Escrig A, et al. Bronquiolitis folicular asociada a inmunodeficiencia común variable. Arch Bronconeumol. 2013;49:166–8.