Obstructive sleep apnea (OSA) is a common disorder1 eliciting sympathetic alterations and intermittent hypoxia (IH) resulting in oxidative stress and inflammation. As a result, OSA has been linked to enhanced cardiovascular (CV) disorders and hypercoagulability,2 endothelial function, intima-media thickness, and high blood pressure.3
Prostanoids (PG) are products of arachidonic acid catabolism by cyclooxygenase (COX) isoenzymes COX-1 and COX-2. Among PG, Thromboxane (TXA2) and Prostacyclin (PGI2) are known for their role as regulators of vascular tone, remodeling and angiogenesis. TXA2 is mainly generated by platelets through COX-1 and quickly metabolized into Thromboxane B2 (TXB2). TXA2 induces platelet activation, vasoconstriction, and vascular smooth muscle cell proliferation. On the other hand, PGI2 mostly depends on endothelial COX-2 and prostacyclin synthase enzymes. PGI2 is metabolized into 6-keto Prostaglandin F1α (6-ketoPGF1α). PGI2 inhibits platelet aggregation and vasoconstriction. Therefore, TXA2 and PGI2 have antagonist properties and are both excreted in urine and plasma.4 Aspirin (acetyl salicylic acid, ASA) is a non-selective COX inhibitor with beneficial anti-thrombotic effects by inhibiting the release of TXA2. Although ASA can also inhibit the synthesis of PGI2 which has anti-thrombotic effect, more pronounced inhibition of TXA2 versus PGI2 has been detected in humans after low-dose ASA.4
Recently, our group reported that pre-atherosclerotic aorta remodeling induced by chronic IH mimicking OSA in mice can be prevented by ASA treatment.5 We here hypothesize, that ASA preventive effects are related to its capacity to inhibit COX-1 and COX-2 pathways. Thus, the aim of the present study is to characterize TXA2 and PGI2 overnight change according to OSA severity, and to investigate the effect of ASA treatment in this overnight change.
We conducted an observational pilot study approved by the Hospital Clinic Ethics Board including 52 patients with OSA suspicion consecutively referred to our sleep laboratory. Either polysomnography or respiratory polygraph was used for diagnosis. All studies were analyzed following the AASM rules6 and divided by apnea-hypopnea index (AHI) severity (low ≤30events/h and high >30events/h) and ASA prescribed as regular medication: low AHI group (n=27): 5/27 with ASA; and high AHI group (n=25): 10/25 with ASA. Patients on other nonsteroidal anti-inflammatory treatments were excluded. Patients data were: 73.1% male, 58.0±12.3 yr old, body mass index (BMI) 28.9±4.6kg/m2, apnea hypopnea index (AHI) 29.9±20.5events//h, oxygen desaturation index 3% (ODI3%), 26.0±20.2events/h.
To assess overnight changes in PG metabolites, urine samples were collected right before patients went to sleep (night) and just after awakening (morning), and immediately stored at −80°C. Subsequently, PG determinations were conducted using Elisa kits for: 11-dehydro Thromboxane B2 (TXB2) and 6-keto-prostaglandin F1α (6-ketoPGF1α) (Cayman Chemical, Ann Arbor, Michigan, USA). Variables across groups were compared with Wilcoxon matched-pairs signed-ranks test and Wilcoxon-Mann–Whitney test. Significance level was set at p=0.05.
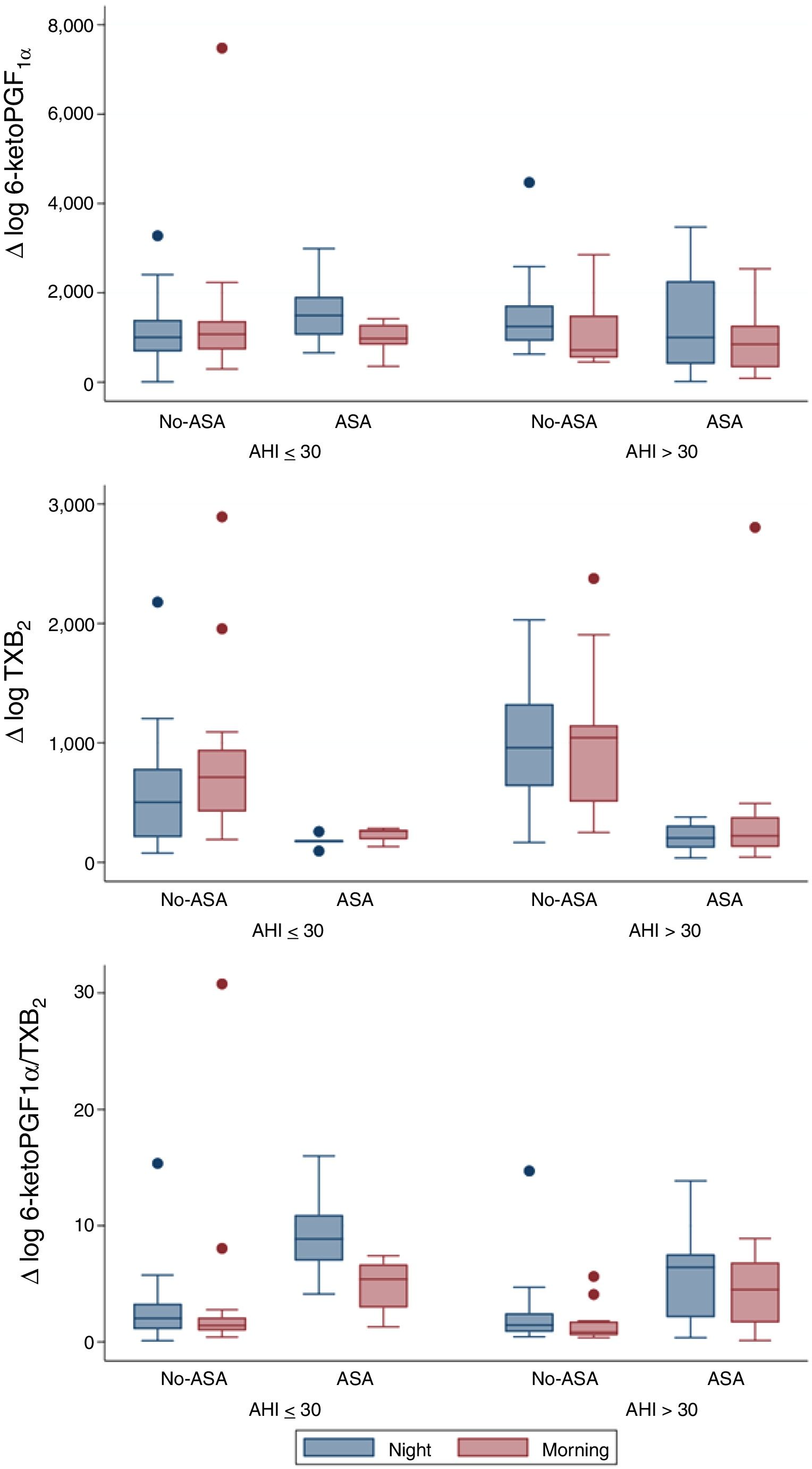
From a total of 52 patients, PG values were measured at the beginning and at the end of the night, in order to evaluate overnight changes according to AHI groups and ASA prescription (Fig. 1). Urinary 6-keto-PGF1α night values were higher in the high AHI than in the low AHI group, but surprisingly they significantly decreased overnight in patients with high AHI levels (n=25; p=0.006) and no-ASA treatment (n=15/25; p=0.006), while in patients on ASA treatment (n=10/25), 6-keto-PGF1α levels drop was not significant. On the other hand, TXB2 increased overnight on both AHI groups, but only significantly in the low AHI group (n=27; p=0.015). As expected, TXB2 was lower in all ASA-treated patients (n=15) and non-significant overnight changes were related to ASA. 6-keto-PGF1α/TXB2 ratio was significantly decreased overnight in both AHI groups (p<0.05), with no differences between AHI severity groups. 6-keto-PGF1α/TXB2 ratio drop remained significant in patients with high AHI and no-ASA treatment (n=15/25; p=0.008). A regression model resulted in similar findings when adjusting by age, gender and BMI.
Our study demonstrated that in severe OSA patients there is a significant overnight drop of PGI2 metabolite (6-keto-PGF1α) in comparison to patients with lower AHI. Meanwhile, TXB2 increased in both groups and resulted in a 6-keto-PGF1α/TXB2 decreased ratio. The results previously reported in the literature were controversial. In accordance with our findings, urinary excretion of PG metabolites in OSA patients suggested a decreased production of dilatory (PGI2) versus constrictor PG (TXB2) expressed by a decreased PGI2/TXB2 ratio.7 Nevertheless, Kimura et al. found a compensatory increase in dilatory PG.8 And more recently Mejza et al. observed that 6-keto-PGF1α urine and serum concentrations were significantly higher in OSA patients when compared to controls.9 Though, TXB2 levels in urine and serum were not significantly different between groups,8 concurrently with our sample. Beaudin et al. assessed IH acute effect in healthy patients (n=12) mimicking severe OSA and PG were unaffected, but these authors found elevated TXA2 levels between in OSA patients compared to the healthy basal levels.10 However, these results must be regarded with caution due to the very small number of patients included in those studies.7–10
Therefore, considering the activation of the TXA2-pathway in OSA patients, particularly with CV comorbidities11 and taking onto account CPAP failure to reduce CV risk12 and TXA2 metabolites excretion,11 we suggest that targeting the COX-1-pathway could represent an alternative strategy to prevent or delay the deleterious CV consequences linked to OSA.13 Yet, in our study regular treatment with ASA had no significant effect over overnight TBX2 increase, probably because the TBX2-pathway was already under ASA inhibition and the limitations of a small sample of patients treated with ASA (n=15). Discrepancies between clinical studies and our murine model5 could also be explained by the greater severity of IH in animal models compared to OSA patients and other contributing factors such as BMI and comorbidities in patients.13 Since this was not an interventional study, the patients ASA treatment could be associated to CV comorbidities or risk factors. Surprisingly, the present study shows that the expected up-regulation of the COX-2 pathway resulting in an increase in the release of protective PGI2 does not take place during the repetitive overnight episodes of hypoxia in OSA patients. Whether this apparent anomaly contributes to the deleterious CV effects of OSA remains to be clarified.
Although the limited number of patients in this pilot study does not allow us to derive solid conclusions with regards to clinical practice, it provides a proof of concept suggesting the interest of further research in larger samples, since it could open new approaches for OSA-CV risk management.
FundingThis work was supported by Premi Fi de Residència “Emili Letang”, SEPAR and SES.
The authors wish to thank JM. Montserrat, R. Farré, X. Alsina and MA. Negrín for their assistance.