The understanding of physicians and the skill of patients in the use of inhalers continues to be inadequate.
ObjectiveThe external validation, by an expert panel, of practical clinical recommendations that had been developed in order to improve the knowledge and understanding of correct inhaled therapy use.
MethodsAfter a bibliographic review about inhaled therapies, 40 clinical recommendations were proposed. A two-round modified Delphi consensus was used to compare the opinions of a panel of 59 experts about the recommendations, which were grouped into 8 areas: general aspects (4), inhaled drugs (9), pressurized metered-dose and spacer inhalers (6), powder inhalers (4), nebulizers (3), devices for mechanical ventilation (3), inhalers for children (5) and issues related with compliance and education (6).
ResultsAfter the first round of the consensus panel, 35 of the 40 recommendations analyzed were accepted. At the end of round 2, agreement was reached in 39 (97.5%). In 8 (20%), the consensus was unanimous (100%). Item 14 was deleted from the recommendations as consensus was not reached.
ConclusionsThe external validation by experts in inhaled therapy found a high level of agreement with the clinical recommendations proposed. This consensus provides a tool that could contribute to the improved use of inhalers in our country in the future.
El conocimiento de los profesionales y la destreza de los pacientes en el uso de los dispositivos inhalados continúa siendo deficiente.
ObjetivoValidación externa por un grupo de expertos de unas recomendaciones clínicas prácticas elaboradas para la mejora del conocimiento y del uso de la terapia inhalada.
MétodosTras una revisión bibliográfica sobre terapia inhalada se propusieron 40 recomendaciones clínicas. Se utilizó el método Delphi modificado en dos rondas para contrastar las opiniones de un panel de 59 expertos sobre dichas recomendaciones. Estas estaban agrupadas en 8 áreas: aspectos generales (4), fármacos inhalados (9), cartucho presurizado y cámaras espaciadoras (6), inhaladores de polvo (4), nebulizadores (3), dispositivos para ventilación mecánica (3), inhaladores para el niño (5) y aspectos relacionados con el cumplimiento y la educación (6).
ResultadosTras la primera ronda del panel se apreció un consenso en la aceptación de 35 de las 40 recomendaciones analizadas. Al final de la segunda ronda se alcanzó el acuerdo en 39 (97,5%). En 8 (20%) el consenso fue por unanimidad (100%). El ítem 14 fue suprimido de las recomendaciones al no alcanzarse el consenso.
ConclusionesLa valoración externa por expertos en terapia inhalada constató un elevado nivel de acuerdo con las recomendaciones clínicas propuestas. Este consenso proporciona un instrumento que podría contribuir a la mejora futura en el uso de los inhaladores en nuestro ámbito.
The use of aerosol drugs in the treatment of respiratory disease has become widely generalized since their introduction in the 1970s. Inhaled administration is today the method of choice for drugs that need to act directly on the bronchial tree, particularly bronchodilator and anti-inflammatory agents. Consequently, standard clinical practice guidelines recommend the use of inhaled therapies for the treatment of obstructive respiratory diseases.1–3 Their main drawback is that a large proportion of patients who use them do so incorrectly, which can cause a lack of therapeutic efficacy and insufficient control of the disease.4,5 This requires clinicians to make an extra effort in order to educate and adequately train patients in the correct use of inhalers. All the clinical practice guidelines for asthma and COPD dedicate special attention to said activity, which is a basic axis of their education programs.1–3 As medical professionals are responsible for the education of their patients, this requires the physicians and nurses who are usually involved in the clinical management of respiratory patients to be familiarized with the details of inhaled therapies and devices for their administration. Nevertheless, the reality is disappointing. Several studies6,7 done over the last 20 years have consistently shown that the majority of doctors who prescribe inhalers, regardless of the type of device considered, were unaware of their correct usage. And what is even worse is that it has recently been confirmed that said lack of awareness does not seem to have improved recently8–10 despite the educational efforts made by scientific societies and workgroups to improve the situation.11,12
In a recent survey of 1514 Spanish doctors from different specialties, it was confirmed that up to 86% of those interviewed showed an insufficient level of knowledge about inhaled therapy.10 Given the severity and the persistence of the deficiencies observed, the members of the Scientific Committee of said study (made up of a multidisciplinary group of physicians specialized in the subject—Annex 1) decided to promote an educational project in order to improve knowledge and understanding of inhaled therapies and inhalers among Spanish medical professionals: the Inhaled Therapy Project. As proof that this problem is also current on an international level, said initiative coincided in objective and in time with the recent publication of the consensus report by the European Respiratory Society and the International Society for Aerosols in Medicine (ERS/ISAM Task Force Report),13 which updates global understanding of aerosol use. Among other actions, the Inhaled Therapy Project prepared a specific monographic document about the subject,14 which included the recommendations for clinical practice made by the authors of each chapter. The aim of the Consensus Statement is to present information to Spanish medical professionals about the appropriate management of inhaled therapies that may lead to future improvement in the treatment provided by both physicians and nursing staff. This present study was created with the objective to validate the recommendations with the Delphi method by a group of experts who did not participate in drafting the aforementioned monographic document.
Material and MethodsDesignA study designed to validate a consensus statement entailing a group of practical recommendations for inhaled therapies and inhalers, using the modified Delphi method in two rounds with an external panel of experts.
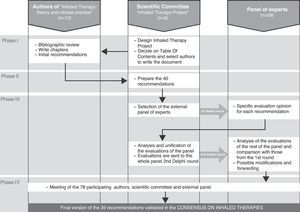
Phases of the ConsensusAs summarized in Fig. 1, the consensus and its validation were developed in four correlative phases:
- •
Phase I. Preparation of the initial draft of the recommendations. After the constitution of the Scientific Committee of the Inhaled Therapy Project (Madrid, January 2010), objectives were set and a plan of action was designed, among these being the publication of a specific monographic document, Inhaled Therapy: theory and clinical practice.14 The table of contents was decided upon and the authors were selected (Annex 1) for each chapter. In the selection process, the most important criteria were those of scientific quality and personal experience in the chapter or sub-chapter assigned. In addition, the authors were requested to include at the end of each chapter some practical recommendations, based on evidence or scientific publications.
- •
Phase II. Preparation of the initial recommendations (July 2010). As described further ahead, the recommendations from the monographic document were synthesized into the 40 items of the consensus. With these, the questionnaire was created to be later used in phase III of the external validation using the Delphi method.
- •
Phase III. External validation by a multidisciplinary expert panel of the 40 initial recommendations. The panel was made up of 59 experts (Annex 1) who had not previously participated as authors of the monographic document. The validation was done with the modified Delphi method in two rounds. The panelists were sent the questionnaire in two separate rounds: after the first round, their opinions were compiled and processed, and a report of these was sent to all the participants before the second round. The fieldwork took place during a time period of 6 weeks in the months of September and October of 2010, and e-mail was used to distribute and receive the questionnaire forms.
- •
Phase IV. Final consensus of the recommendations after their external validation. The results of the Delphi survey were analyzed, and these were discussed in a meeting (Madrid, December 2010) to which all the 78 participants in the consensus were invited (authors, external reviewers from the Delphi analysis and members of the Scientific Committee).
Flowchart of the four phases involved in preparing the recommendations of the Consensus on Inhaled Therapies. In phase I, the monographic document “Inhaled Therapies: theory and clinical practice”14 was published. In doing so, the 13 authors were asked to propose practical recommendations for each of the chapters. In phase II, the six members of the Scientific Committee of the Project synthesized the previous recommendations into the 40 items of the consensus. In phase III, 59 experts who had not participated in the monographic document carried out the validation of 39 of the 40 proposed recommendations, using the modified Delphi method. And in phase IV an analysis was performed of the results of the Delphi survey, which were discussed in a meeting of the 78 participants in the four phases of the consensus.
The panel was selected by the Scientific Committee. The inclusion criteria used for their selection were: proven clinical experience in asthma or COPD patient care and research as well as a leading role in education or research about inhalers and inhalation techniques. The only exclusion criterion used was having participated in the writing of the monographic documents. For the identification of panel experts, a “snowball” strategy was used, starting with the personal contacts of the members of the Committee, who then in turn proposed new candidates who met the participation criteria from their professional setting. After this process, 60 professionals were contacted by a letter inviting them to participate in the panel, 59 of whom accepted (Annex 1).
The Delphi MethodIn the present study, the modified Delphi method was used,15 which is a structured professional consensus technique that is a variation of the original procedure developed by Dalkey et al. at Rand Corporation.16,17 It maintains its main advantages over other alternative techniques (such as consensus conferences, nominal groups or unstructured meetings) and resolves some of the limitations.18 Nevertheless, some drawbacks of the modified Delphi method are the difficulty to explain or clarify the personal assessments expressed, the need for a strong involvement of the participants in the Project in order to achieve high response rates and the fact that it is not a method free from the possible influences of its promoters (in selecting the panel of experts and in discussing the results). In order to control these risks, this present study was planned and co-directed by a multi-center research team with different origins and interests, all of whom followed systematized procedures and objectives for the selection of panelists (“snowball” method)19 and in the statistical analysis and interpretation of the results.20,21
Development of a Questionnaire Used in the Delphi Survey of the StudyDuring phase II, the Scientific Committee that promoted the project, with the collaboration of an external methodological advisor, selected the content of the Delphi questionnaire. This was based on an indeterminate number of items that had previously been requested from the authors of each of the chapters of the monographic document14 on which the Project was based.
The preliminary list of items received from each author underwent a process of selection, revision and in some cases adaptation, until a version was achieved that was satisfactory for all the members of the committee. Each item that was finally chosen was an asseveration (affirmative or negative) that dealt with professional criteria or a clinical recommendation for improving the use of inhaled therapy.
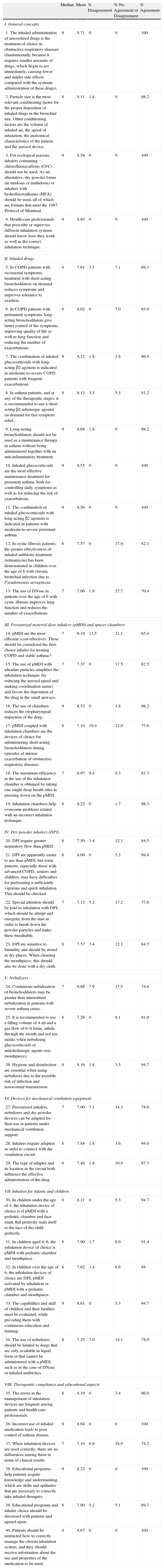
The final version of the questionnaire included 40 items (Table 1), grouped in the following 8 subject areas: general concepts (4 items), inhaled medication (9 items), pressurized metered-dose inhalers (pMDI) and spacer inhalers (6 items), dry powder inhalers (DPI) (4 items), nebulizers (3 items), devices for mechanical ventilation equipment (3 items), inhalers for infants and children (5 items), and therapeutic compliance and educational aspects (6 items).
Recommendations of the Multidisciplinary Consensus on Inhaled Therapies and the Results Obtained for Each of the Items of the Delphi Questionnaire Evaluated by the External Panel of Experts. In the End, Item 14 Was Withdrawn Because No Consensus Was Reached.
| Median | Mean | % Disagreement | % No Agreement or Disagreement | % Agreement | |
| I. General concepts | |||||
| 1. The inhaled administration of aerosolized drugs is the treatment of choice in obstructive respiratory diseases (fundamentally because it requires smaller amounts of drugs, which begin to act immediately, causing fewer and milder side effects compared with the systemic administration of these drugs). | 9 | 8.71 | 0 | 0 | 100 |
| 2. Particle size is the most relevant conditioning factor for the proper deposition of inhaled drugs in the bronchial tree. Other conditioning factors are the volume of inhaled air, the speed of inhalation, the anatomical characteristics of the patient and the aerosol device. | 8 | 8.11 | 1.8 | 0 | 98.2 |
| 3. For ecological reasons, inhalers containing chlorofluorocarbons (CFC) should not be used. As an alternative, dry powder forms (in unidosis or multidosis) or inhalers with hydrofluoroalkanes (HFA) should be used, all of which are formats that meet the 1987 Protocol of Montreal. | 9 | 8.58 | 0 | 0 | 100 |
| 4. Health-care professionals that prescribe or supervise different inhalation systems should know how they work as well as the correct inhalation technique. | 9 | 8.93 | 0 | 0 | 100 |
| II. Inhaled drugs | |||||
| 5. In COPD patients with occasional symptoms, treatment with short-acting bronchodilators on demand reduces symptoms and improves tolerance to exertion. | 8 | 7.61 | 3.5 | 7.1 | 89.3 |
| 6. In COPD patients with permanent symptoms, long-acting bronchodilators give better control of the symptoms, improving quality of life as well as lung function and reducing the number of exacerbations. | 8 | 8.02 | 0 | 7.0 | 93.0 |
| 7. The combination of inhaled glucocorticoids with long-acting β2 agonists is indicated in moderate-to-severe COPD patients with frequent exacerbations. | 8 | 8.12 | 1.8 | 1.8 | 96.4 |
| 8. In asthma patients, and at any of the therapeutic stages, it is recommended to use a short-acting β2-adrenergic agonist on demand for fast symptom relief. | 9 | 8.12 | 3.5 | 5.3 | 91.2 |
| 9. Long-acting bronchodilators should not be used as a maintenance therapy in asthma without being administered together with an anti-inflammatory treatment. | 9 | 8.68 | 1.8 | 0 | 98.2 |
| 10. Inhaled glucocorticoids are the most effective maintenance treatment for persistent asthma, both for controlling daily symptoms as well as for reducing the risk of exacerbations. | 9 | 8.55 | 0 | 0 | 100 |
| 11. The combination of inhaled glucocorticoids with long-acting β2 agonists is indicated in patients with moderate-to-severe persistent asthma. | 9 | 8.56 | 0 | 0 | 100 |
| 12. In cystic fibrosis patients, the greater effectiveness of inhaled antibiotic treatment (tobramycin) has been demonstrated in children over the age of 6 with chronic bronchial infection due to Pseudomonas aeruginosa. | 8 | 7.57 | 0 | 17.9 | 82.1 |
| 13. The use of DNase in patients over the age of 6 with cystic fibrosis improves lung function and reduces the number of exacerbations. | 7 | 7.06 | 1.9 | 27.7 | 70.4 |
| III. Pressurized metered-dose inhalers (pMDI) and spacer chambers | |||||
| 14. pMDI are the most efficient (cost-effective). These should be considered the first-choice inhaler for treating COPD and stable asthma.a | 7 | 6.19 | 13.5 | 21.1 | 65.4 |
| 15. The use of pMDI with ultrafine particles simplifies the inhalation technique (by reducing the aerosol speed and making coordination easier) and favors the deposition of the drug in the small airways. | 7 | 7.37 | 0 | 17.5 | 82.5 |
| 16. The use of chambers reduces the oropharyngeal impaction of the drug. | 9 | 8.52 | 0 | 1.8 | 98.2 |
| 17. pMDI coupled with inhalation chambers are the devices of choice for administering short-acting bronchodilators during episodes of intense exacerbation of obstructive respiratory diseases. | 8 | 7.10 | 10.4 | 12.0 | 77.6 |
| 18. The maximum efficiency in the use of the inhalation chamber is obtained by taking one single deep breath after la pressing down on the pMDI. | 7 | 6.97 | 9.4 | 9.3 | 81.3 |
| 19. Inhalation chambers help overcome problems related with an incorrect inhalation technique. | 8 | 8.22 | 0 | 1.7 | 98.3 |
| IV. Dry powder inhalers (DPI) | |||||
| 20. DPI require greater inspiratory flow than pMDI. | 8 | 7.50 | 3.4 | 12.1 | 84.5 |
| 21. DPI are apparently easier to use than pMDI, but some patients, especially those with advanced COPD, seniors and children, may have difficulties for performing a sufficiently vigorous and quick inhalation. This should be checked. | 8 | 8.00 | 0 | 5.2 | 94.8 |
| 22. Special attention should be paid to inhalation with DPI, which should be abrupt and energetic from the start in order to break down the powder particles and make these breathable. | 7 | 7.12 | 5.2 | 17.2 | 77.6 |
| 23. DPI are sensitive to humidity and should be stored in dry places. When cleaning the mouthpiece, this should also be done with a dry cloth. | 8 | 7.57 | 3.4 | 12.1 | 84.5 |
| V. Nebulizers | |||||
| 24. Continuous nebulization of bronchodilators may be greater than intermittent nebulization in patients with severe asthma crises. | 7 | 6.88 | 7.9 | 17.5 | 74.6 |
| 25. It is recommended to use a filling volume of 4ml and a gas flow of 6–8l/min, inhale through the mouth and not use masks when nebulizing glucocorticoids or anticholinergic agents (use mouthpiece). | 8 | 7.26 | 0 | 8.1 | 91.9 |
| 26. Hygiene and disinfection are essential when using nebulizers due to the possible risk of infection and nosocomial transmission. | 8 | 8.16 | 1.8 | 3.5 | 94.7 |
| VI. Devices for mechanical ventilation equipment | |||||
| 27. Pressurized inhalers, nebulizers and dry powder devices can be adapted for their use in patients under mechanical ventilation support. | 7 | 7.00 | 7.1 | 14.3 | 78.6 |
| 28. Inhalers require adapters in order to connect with the ventilation circuit. | 8 | 7.88 | 1.8 | 3.6 | 94.6 |
| 29. The type of adapter and its location in the circuit both influence the effective administration of the drug. | 8 | 7.49 | 1.8 | 10.9 | 87.3 |
| VII. Inhalers for infants and children | |||||
| 30. In children under the age of 4, the inhalation device of choice is el pMDI with a pediatric chamber and face mask that perfectly seals itself to the face of the child perfectly. | 8 | 8.21 | 0 | 5.3 | 94.7 |
| 31. In children aged 4–6, the inhalation device of choice is pMDI with pediatric chamber and mouthpiece. | 8 | 7.90 | 1.7 | 6.9 | 91.4 |
| 32. In children over the age of 6, the inhalation devices of choice are DPI, pMDI activated by inhalation or pMDI with a pediatric chamber and mouthpiece. | 8 | 7.62 | 3.4 | 8.6 | 88 |
| 33. The capabilities and skill of children and their families must be evaluated, while providing them with continuous education and training. | 9 | 8.61 | 0 | 5.3 | 94.7 |
| 34. The use of nebulizers should be limited to drugs that are only available in liquid form or that cannot be administered with a pMDI, such as in the case of DNase or inhaled antibiotics. | 8 | 7.25 | 7.0 | 14.1 | 78.9 |
| VIII. Therapeutic compliance and educational aspects | |||||
| 35. The errors in the management of inhalation devices are frequent among patients and health-care professionals. | 8 | 8.19 | 0 | 3.4 | 96.6 |
| 36. Incorrect use of inhaled medication leads to poor control of asthma disease. | 9 | 8.64 | 0 | 0 | 100 |
| 37. When inhalation devices are used correctly, there are no differences among them in terms of clinical results. | 7 | 7.10 | 6.9 | 18.9 | 74.2 |
| 38. Educational programs help patients acquire knowledge and understanding, which are skills and aptitudes that are necessary to correctly take inhaled therapies. | 9 | 8.22 | 0 | 0 | 100 |
| 38. Educational programs and inhaler choice should be discussed with patients and agreed upon. | 8 | 7.90 | 5.2 | 5.1 | 89.7 |
| 40. Patients should be instructed how to correctly manage the chosen inhalation system, and they should receive information about the use and properties of the medication to be used. | 9 | 8.67 | 0 | 0 | 100 |
One type of scale was decided upon to answer all the questions, the ordinal nine-point Likert-type scale (minimum 1, complete disagreement; and maximum 9, complete agreement), according to the format developed by UCLA-Rand Corporation for the method for evaluating the appropriate use of health-care technology.16,17 The response categories are reported with definitions in three regions (1–3=“disagree”; 4–6=neither agree nor disagree”; 7–9=agree”). In each case, the survey participants were able to detail their own particular opinion, choosing between the three points of each region. The questionnaire offered the participants the possibility to include free observations and there was a final section for them to propose new questions if they so desired. If one of the questions was not completed as the panelist him/herself did not feel qualified in the matter, it was treated as a lost case in the statistical analysis.
Analysis and Interpretation of the ResultsIn order to analyze the group opinion and the type of consensus reached for each question, we used the position of the mean group score as well as the “level of agreement” reached by the survey participants, according to the criteria that are detailed below.
An item was considered to have a consensus when there was agreement of opinion on the panel. This means that experts who scored outside the three-point region ([1–3], [4–6], [7–9]) of the mean represented less than one-third of those surveyed. In any case, the value of the median determined the group consensus reached: majority “disagreement” with the item if the mean was equal or less than 3, or majority “agreement” with the item if the mean was equal to or higher than 7. The cases in which the mean was in the 4–6 margin were considered “undecided” items for a representative majority of the group.
Conversely, it was established that there was criterion for “disagreement” in the panel when the scores of one-third or more of the panelists were in the [1–3] region and another third or more in the [7–9] region. The remaining items that showed neither agreement nor disagreement were considered to have an “undetermined” level of consensus.
All the items in which the group did not reach a manifest consensus either in favor of or against the question posed (the undecided items, those in which disagreement was observed and those that showed an indeterminate level of consensus) were proposed for reconsideration by the panel in the second Delphi round. Also sent for reevaluation were those items in which there was an observed high dispersion of opinions among those who were surveyed with an interquartile range equal to or higher than 4 points (range of scores contained between values p25 and p75 of the distribution).
Between the two rounds, the panelists were informed in detail about the distribution of the group responses in the first survey (using bar graphs). Comments and clarifications given by each participant were also provided. After reviewing this information, the participants were asked to re-assess the items that did not reach consensus in the first round.
After the second round of the survey, identical criteria were applied in order to distinguish the items that reached definitive consensus from those in which it was not possible to unify the experts’ criteria. In order to provide a graphic comparison between items, the average score of the panelists was calculated for each question, along with the 95% confidence interval. The more extreme the average score of an item (meaning closer to either 1 or 9), the clearer the consensus reached for each item posed, albeit in agreement or disagreement. The items which did not reach a consensus after completing the process described were analyzed descriptively in order to determine whether the situation were due to persisting disagreement in criteria or rather to doubt among the majority of the panel regarding an item (vote between 4 and 6).
ResultsThe mean age of the 59 expert panelists was 50.3, with a majority of males (83%). All of them were clinicians who were actively working in public health-care services (simultaneously in private practice in 16% of the cases), mostly hospitals (82%), with an average of 21.6 years of professional experience. They represented all the autonomous communities (provinces) of the country and came from different specialties, including pulmonology (85%), allergology, internal medicine, family medicine and pediatrics.
The experts consulted completed the two rounds of evaluation and they proposed no new items. In the first round, consensus was reached for 35 of the 40 recommendations analyzed according to the pre-established evaluation criteria. The consensus for these 35 items was of group agreement (acceptance) with the questions posed. Out of the 5 remaining items that were proposed for reconsideration by the experts in the second round, a consensus was reached for 4 more, also with group agreement with the item in question. Considered as a whole, the panel reached consensus for 97.5% of the recommendations proposed. Among the observed results were that 8 (20%) of the items reached unanimous consensus (100% acceptance) from the experts. Out of these 8 items, 3 belonged to the “general aspects” group (items 1, 3 and 4), 2 to “inhaled drugs” (items 10 and 11) and 3 to the “aspects related with compliance and education” (items 36, 38 and 40).
Table 1 compiles the values obtained in each of the items or proposals made with their corresponding statistics, indicating for each item the median and mean scores as well as the distribution of the participants in each of the consensus regions (agreement, undecided or disagreement with the item).
There was only one item, number 14, which did not reach a consensus, either for agreement or disagreement. After the two rounds, it still continued to have an indeterminate level of consensus among the panelists (lack of agreement or disagreement according to pre-established criteria). Said item affirmed that “pressurized metered-dose inhalers (pMDI) are the most efficient (cost-effective) and, consequently, should be considered the inhaler of choice”. Although this was an opinion shared by most of the panelists (65.4%), a small but significant subgroup of experts (13.5%) manifested their opinion to the contrary, while the remaining participants (21.1%) declared having doubts.
DiscussionThe external assessment by experts in inhaled therapies from the different specialties that have made up the panel in the present study shows a high level of agreement with the proposed recommendations (97.5%). This external acceptance gives credit to the previous task of recompilation carried out by the writers of the document “Inhaled Therapy: theory and clinical practice” and to the task of agglutination and synthesis by the members of the Executive Committee of the project who drafted the recommendations. It should be highlighted that the majority (35 out of 40) of these reached consensus in the first round of the Delphi survey, and that the scores of the evaluations emitted by the experts for each of the items were at very high levels. In fact, for 8 recommendations the agreement was unanimous. In general terms, the high level of agreement observed is situated above what is usually reported in studies with similar characteristics.21,22 And, this is of special interest when, in this present analysis, the panel of experts was comprised of professionals from different origins, both corporate and geographical, with different responsibilities and health-care activities.
The results of this study endorse the recommendations of experts that make up the present Consensus on Inhaled Therapies. In our setting, there is no similar precedent, and therefore this consensus of clinical recommendations is the first text on inhaled therapies and inhalation devices to be completed in Spain. The high number of participating experts (78) in the Project, their prestige and multidisciplinary origin contribute to the credibility of the document. The high degree of consensus observed among the members of the panel in the majority of the items examined leads us to believe that these clinical recommendations and criteria are supported by the unanimous professional opinion of Spanish experts. This aspect, in addition to the endorsement of the Consensus, may be considered an interesting contribution to be diffused and followed by health-care professionals. This would promote its practical application in reducing the unjustified variability found among these professionals. Among the causes of the poor compliance with clinical practice guidelines are the different criteria and lines of action seen from specialty to specialty, causing excessive variability in clinical practice. For this reason, it is possible that in the future the Consensus may become a common reference text for inhaled therapies in our setting, which physicians may turn to and cite in order to support his/her own considerations or recommendations, regardless of the specialty in question. The initiative concurs with other similar initiatives recently done in the United States12 and in Europe.13
The result of the non-consensus observed with item 14 (about whether pressurized cartridges are the most cost-effective devices available) merits comment. First of all, the lack of consensus does not mean that the item was rejected, because in that case we could have witnessed agreement in its being rejected. Instead, the fragmentation observed in the responses given by the experts (65.4% agreed, 13.5% disagreed and 21.1% were on the fence) meant that consensus was not reached after two rounds. Second, this lack of consensus neither invalidates nor rules out its affirmation; it may simply translate either the lack of scientific evidence to promote a categorical opinion, or that such evidence is too heterogeneous, with conflicting results seen in the literature. As a consequence, the situation leads one to reflect upon the need to develop well-designed cost-efficiency studies that could shed light on the question. This would allow us to later, in light of future results, unify professional opinions, either by accepting or rejecting the initial pending recommendation.
Considerable efforts have been made in education in recent years in order to improve the level of knowledge about aerosols and their correct administration among health-care professionals in our country. In spite of this, the situation unfortunately continues to be less than optimal,10 and this circumstance seems to be universal.8,9 The causes of this phenomenon should be investigated and new educational strategies should be designed and implemented to efficiently resolve this shortcoming. In this context, and with the ambitious intention of making a contribution to mitigate these mentioned deficiencies, the Inhaled Therapy Project was born. It is basically an educational initiative that is locally based in the Spanish setting, whose aim is to update the understanding in this material among health-care professionals. It unites different complementary actions which have been occurring since 2010. In addition to publishing the aforementioned monographic document entitled “Inhaled Therapies: theory and clinical practice”14 and the OPTIM-Test study,10 the Project has designed, together with the sponsor of the initiative, a series of training meetings and debates in smaller groups of professionals from different specialties distributed all over Spain during 2011 and 2012. Among the objectives of these meetings, the most important, without a doubt, is to disseminate the 39 recommendations embodied in the Consensus on Inhaled Therapies for clinical hat were validated in the present study. We are certain that all these efforts made will result in an improvement in the training and performance of our professionals, which will consequently translate into better patient use of inhalers, ultimately providing them optimal control of their disease and a better quality of life. This is the raison d’être as well as the main objective of the Inhaled Therapy Project.
Among the possible design limitations of this study is the method used for selecting the members of the panel of experts who participated in the validation of the consensus recommendations. Even though there could be a certain (but not deliberate) bias of choice, there was no better alternative, particularly if what we intended was for it to be comprised of experts. On the contrary, among its strengths are the high number of participants, their territorial representation and a certain multidisciplinary participation. Although most were pulmonologists, other specialists also participated from the areas of allergology, primary care and internal medicine.
In short, the study demonstrated the validation, with excellent levels of agreement among experts, of a group of practical recommendations created to improve the understanding and use of inhaled therapies. The resulting test is the first Consensus on Inhaled Therapies done in our country to date. The diffusion of said document could contribute to increasing the degree of knowledge of health-care professionals about the use of inhalers for treating obstructive respiratory diseases.
FundingThe study received unconditional help from Chiesi Farmaceutici, which did not intervene in the data collection, analysis or interpretation of the results or in the drafting of the manuscript.
Conflict of InterestsThe authors present no conflict of interests with regards to this article.
We would like to thank all the participants in the Inhaled Therapy Project, particularly the experts who provided an external evaluation of the recommendations in the Consensus on Inhaled Therapies of the Delphi survey and the authors of the chapters of the monographic document “Inhaled Therapies: theory and clinical practice”, for their valuable contribution. We would like to acknowledge the team of the Unidad de Investigación Clínico-Epidemiológica del Grupo Luzán 5, S.A. for their professionalism. Thanks also to Chiesi for sponsoring the Inhaled Therapy Project, especially to Dr. Cristina Murio of their Medical Department for her constant support and personal commitment.
Myriam Calle Rubio, Jesús Molina París, Vicente Plaza Moral, Santiago Quirce Gancedo, Joaquín Sanchis Aldás and José Luis Viejo Bañuelos.
Eduardo Calvo Corbella, Enrique Cimas Hernando, María Luz García García, Jordi Giner Donaire, Dolores Hernández Fernández de Rojas, José María Ignacio García, Vicente Macián Gisbert, Luís Maíz Carro, José María Olaguíbel Ribera, Miguel Perpiñá Tordera, Gustavo Rodrigo, Juan Miguel Sánchez Nieto and Joan Serra Batlles.
Ramón Agüero Balbín, Carlos Almonacid Sánchez, Aurelio Arnedillo Muñoz, Santiago Bardagí Forns, Pilar Barranco Sanz, Rafael Blanquer Olivas, Elena Bollo de Miguel, Luis Borderías Clau, Víctor Bustamante Madariaga, José Francisco Carboneros de la Fuente, José Ángel Carretero Sierra, Francisco Casas Maldonado, Eusebi Chiner Vives, Carlos Colás Sanz, Joaquín Costán Galicia, Julio Delgado Romero, Luis Domínguez Juncal, Javier Domínguez Ortega, Luis Manuel Entrenas Costa, María José Espinosa de los Monteros Garde, Javier Fernández de Córdoba, Javier Fernández Sánchez, Alberto Fernández Villar, José Fernando Florido López, Pedro Gamboa Setién, Francisco de Borja García-Cosío Piqeras, Fernando Gómez Ruiz, Jacinto Hernández Borge, Jesús Hernández Hernández, Milagros Iriberri Pascual, María Antonia Juretschke Moragues, Javier Lázaro Polo, María Antonia Llauger Roselló, Eleuterio Llorca Martínez, Tomás Lloret Pérez, Miguel Ángel Lobo Alvarez, Antolín López Viña, Damián Malia Alvarado, Lluis Marqués Amat, Agustín Martínez González, Fernando Masa Jiménez, Carlos Melero Moreno, Alfonso Javier Miranda Páez, María Molina Molina, Luis Molinos Martín, Álvaro Moreno Ancillo, Xavier Muñoz Gall, Francisco Ortega Nogueras, Antonio Peláez Hernández, Gerardo Pérez Chica, Luis Pérez de Llano, Miguel Perpiñá Tordera, César Picado Vallés, Ana María Pueyo Bastida, Joaquín Quiralte Castillo, María Jesús Rodríguez Nieto, Miguel Román Rodríguez, Pilar Sanz Sanz, Pedro J. Simonet Aineto, Alfons Torrego Fernández, Juan Antonio Trigueros Carrero, José L. Trujillo, Luis Guillermo Valdés Cuadrado, Agustín Valido Morales, Carmen Vidal Pan, Carlos Villasante Fernández-Montes, Joaquín Vizcaíno Ricoma and Manuel Vizcaya Sánchez.
Please cite this article as: Plaza V, et al. Validación externa de las recomendaciones del Consenso multidisciplinar sobre Terapia Inhalada. Arch Bronconeumol. 2012;48:189–96.