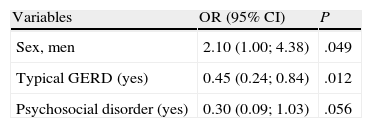
Our objective was to evaluate the association between chronic cough and the variables that could influence the course of the cough in order to develop a profile for coughers with poor response to treatment. In our Chronic Cough Unit, 192 patients were prospectively followed up for 3 months, during which time all the variables that could influence the cough reflex were evaluated and treated. The improvement in cough was evaluated by the response of the patients to a visual analogical scale with scores from 0 to 4, considering 0 as «no changes» and an improvement as a score of 3 or 4. The cough was considered to have little response to treatment if it persisted without any improvement for more than 3 months. Using a multivariate logistic regression model, we input variables that were candidates for being associated with the improvement in cough 3 months later. In the final profile model of the cougher with poor prognosis, three variables remained: sex, typical gastroesophageal reflux and psychosocial disorder. Being male is associated with an improvement in cough 3 months later (OR=2.10, 95%CI 1.00–4.38). However, having gastroesophageal reflux is associated with a reduction in the improvement three months later in 55% (OR=0.45, 95%CI 0.24–0.84), and having a psychosocial disorder reduces the probability for improvement of the cough 3 months later in 70% (OR=0.30, 95%CI 0.09–1.03).
Nuestro objetivo fue evaluar la asociación entre tos crónica y las variables que pudieran incidir en el curso de la tos, con objeto de extraer un perfil del tosedor de peor respuesta. En nuestra unidad de tos crónica 192 pacientes fueron seguidos prospectivamente durante 3 meses, durante los que se valoraron y trataron todas las variables que pudieran influir en el reflejo de la tos. La mejoría de la tos se evaluó por la respuesta del paciente ante una escala visual analógica con puntuaciones de 0 a 4, considerándose el valor 0 como «sin cambios», y como mejoría una puntuación en la escala de 3 o 4. Se consideró tos de escasa respuesta si persistía sin mejoría más allá de los 3 meses. Mediante un modelo de regresión logística multivariante se introdujeron variables candidatas a estar asociadas a la mejoría de la tos a los 3 meses. En el modelo final del perfil del tosedor de mal pronóstico permanecen 3 variables: sexo, reflujo gastroesofágico típico y trastorno psicosocial. Ser hombre está asociado con una mejoría de la tos a los 3 meses (OR=2,10, IC 95%: 1,00-4,38). Sin embargo, presentar reflujo gastroesofágico está asociado con una reducción de la mejoría a los 3 meses en un 55% (OR=0,45, IC 95%: 0,24-0,84), y padecer un trastorno psicosocial disminuye la probabilidad de mejoría de la tos a los 3 meses en un 70% (OR=0,30, IC 95%: 0,09-1,03).
Chronic cough, or cough that persists for more than 2 months, is now considered a manifestation of a hypersensitive cough reflex, and with proper treatment, the said hypersensitivity can decrease.1 Persistent cough interferes with quality of life and has repercussions in daily social life; it can affect sleep and patients occasionally present muscle cramps, side pain, rib fractures and urinary or fecal incontinence and syncopal episodes, although these are rare.
It is known that there are a relatively small number of anatomical locations for cough receptors and afferent nerves that correspond with a clearly defined number of diseases or conditions that could stimulate these areas and result in chronic or persistent cough. On the other hand, an intact cough reflex is crucial in order to maintain the protection of the airway. Thus, when treating chronic cough, the aim should be mitigating the state of hypersensitivity, and treating the aggravating factor that raises the cough reflex threshold which could improve prognosis. An exacerbated cough reflex can be multifactorial in origin, and based on this fact chronic cough is considered a syndrome2,3 in which there may be several phenotypes.4 The objective of this study is to explore which factors are associated with a poorer prognosis and, consequently, whether it is possible to obtain a patient profile with chronic cough and poor response to treatment.
MethodologyDuring an 8-month period at the chronic cough unit at our hospital, 192 patients with chronic cough were seen in a prospective protocol. These patients were sent to our unit due to either limited or no response to the treatment prescribed by their primary-care physicians, internists, ENT specialists, pulmonologists or allergists, and they were consecutively selected for study. Ours is a cross-sectional, observational study of standard clinical practice, and the Ethics Committee at our hospital approved the operating protocol.
For each case, we collected patient data, including: occupation, tobacco habit, previous thoracic or systemic diseases and medication received, with special attention given to angiotensin converting enzyme (ACE) inhibitors and beta-blockers. Likewise, we investigated all the aggravating factors that could influence the cough reflex, and during the first month of follow-up we carried out the tests that were considered necessary to better define these factors. Each aggravating factor that was identified was treated. We placed special emphasis on the diagnosis of gastroesophageal reflux disease (GERD), given the growing interest in the literature about its extraesophageal manifestations, among these chronic cough.5 After three months of treatment, we determined the magnitude of improvement for each patient, according to a visual analogical scale: 0=no improvement; 1=very slight improvement; 2=slight improvement; 3=noticeable improvement; 4=disappearance of the cough. This scale, which is a safe and reproducible measurement,6 was shown to a patient on a 10-cm panel upon which the patient chose his/her state from 0 (no change) to 4 (disappearance of the cough), while scores 1, 2 and 3 represented proportions of successive quarters on the scale in ascending progression of improvement. In our method, the positive evaluation of the cough began in our method with a score of 3, which would represent an improvement of between 75% and 100%, reflecting an improvement that could be considered very good at the very least.
In every case, there were initial chest X-rays and spirometries made available, and we later followed the guidelines for diagnosis and treatment according to the SEPAR protocol,7 with some additional modifications from the 2004 guidelines for chronic cough by the European Respiratory Society.8 All the patients answered questionnaires and tests were done to either confirm or rule out the most frequent aggravating factors in chronic cough, according to the criteria detailed below.
Cough Variant Asthma or Asthmatic CoughDefined by reversible airflow obstruction and a history of episodic wheezing or respiratory difficulty in addition to spirometry with a positive bronchodilator test (variation of FEV1 after 200μg of salbutamol higher than 12% and more than 200ml) and/or a positive methacholine test (PC20 methacoline equal to or less than 8mg/ml) and/or a variability of peak flow greater than 15% in 2 measurements in one day.9
Atopy was defined by total serum IgE>100KU/l and/or positive skin test or specific IgE (InmunoCAP Phadia) to common aeroallergens. The treatment of asthma included inhaled corticosteroids (budesonide, 800μg/day or equivalent).
Eosinophilic BronchitisDefined by the presence in sputum of a percentage of eosinophils equal to or greater than 3%, but with a negative methacoline test.10 The treatment of eosinophilic bronchitis included budesonide (at least 800μg/day or equivalent).
Gastroesophageal RefluxThe diagnosis was confirmed based on several clinical criteria:
- •
A clinical history compatible with esophageal or extraesophageal manifestations of reflux.11 These symptoms are retrosternal burning or regurgitation, and either one is considered a typical esophageal manifestation of GERD. Contrarily, an association with hoarseness, excess mucus or the sensation of a foreign body located in the throat, frequent clearing of the throat and a sensation of a lump in the throat are all associated with extraesophageal or atypical manifestations of GERD. When 3 or more of these symptoms are seen in a chronic cougher, there is suspicion for a diagnosis of cough associated with extraesophageal reflux. In any case, in order to assume a high suspicion of cough associated with reflux, the presence of GERD and/or hiatal hernia should be confirmed by a barium-contrast gastro-esophageal transit study.
If the patient with the diagnosis of chronic cough associated with typical or atypical GERD did not satisfactorily respond to 2 months of anti-reflux therapy, physiological studies of the esophagus were ordered, as recommended by the ERS guidelines.8
- •
Patients were also considered to have cough associated with GERD when the result of the physiological studies of the esophagus was positive. Positivity was taken as ambulatory monitoring of pH (pH-metry) compatible with acid reflux, with a pH<4, more than 4% of the 24h del register, as well as the presence of esophageal dysmotility diagnosed by a pathological manometry. The manometry was considered pathological when, following Leite et al.,12 one or more of the following 4 criteria were met: when the pressure of the lower esophageal sphincter (abdominal component) was <10cmH2O, or rather when the number of unintentional contractions was >30% of the total of a series of swallows of water, or rather the peristaltic compressions were <15cmH2O, or lastly, when double-peak, triple peak or tertiary contractions were detected.
Treatment for GERD included: proton pump inhibitors (PPI) (omeprazole or similar) at a dose of 20 or 40mg when fasting every 12h and advice regarding diet and sleeping positions, with the addition of cinitapride or metoclopramide as stimulants of digestive motility in cases with a limited improvement in cough after one month of treatment with PPI.
Endoscopic findings compatible with laryngopharyngeal reflux (LPR) were evaluated, and LPR was determined if the score was equal to or higher than 5 on a scale of 0–14 points.13
Chronic Cough Syndrome Associated With the Upper AirwayA high probability of rhinosinusitis as a cause of chronic cough was suggested by a combination of symptoms such as frequent clearing of the throat, post-nasal drip, nasal discharge, nasal obstruction or hoarseness, in addition to a computed tomography of the upper airway showing significant alterations.
Cough Induced by Taking Angiotensin-converting Enzyme InhibitorsThe diagnosis was accepted if the cough was resolved after suspending treatment with this medication.
Cough Associated With Psychosocial DisordersWhen there was a strong suspicion of cough associated with a psychosocial disorder (basically anxiety and depression) the patients were sent to the psychiatric department for cognitive therapy.
Exploration ProceduresMeasuring the Exhaled Fraction of Nitric OxideWe used a NIOX MINO device that is adequate for routine clinical practice and which has been shown to be highly reproducible in large population studies.14,15 According to our experience, a value higher than 33 parts per billion (ppb) was considered diagnostic for eosinophilic inflammation of the airway in chronic cough.16 The measurement of the exhaled fraction of nitric oxide (FENO) was done once in the morning, 2h after breakfast.
Spirometry and Reversibility TestSpirometry was done using a compact spirometer by Jaeger, and reversibility was defined as an increase in FEV1 of more than 12% above the baseline value and higher than 200ml, after the inhalation of 200μg of salbutamol.
Bronchial HyperresponsivenessThe methacholine provocation test was done in accordance with the recommendations of the ERS.17 The test was done using the tidal breathing method.
Statistical AnalysisThe sample has been described using absolutes and relative frequencies in the categorical variables, and by using means and standard deviation in the numerical variables. Hypothetical contrasts were done in order to evaluate the association between the variable being studied (improvement in cough in 3 months) and the rest of the variables collected, which we can group into different categories. On one hand are the sociodemographic variables, such as gender, smoking or being diagnosed with a psychosocial disorder. On the other hand are the variables that include potential comorbidities, such as the prescription of ACE inhibitors and obesity. Lastly, we analyzed a series of variables having to do with clinical information, such as the diagnosis of asthma with function testing, diagnosis of upper airway cough syndrome (UACS), diagnosis of typical or atypical GERD, atopy, initial FENO level and the index of endoscopic findings for laryngopharyngeal reflux. The test used for the contrast of hypotheses was either the Chi-squared or the Student's t, as necessary.
A multivariate logistic regression model was carried out with the variables that were candidates for improving cough in 3 months. The variables in the descriptive study were: sex (female/male), functional asthma (in 3 categories: not tested, positive [when at least one of these tests were positive: BD test, BHR test, variability of peak flow] or negative [none of the cited tests was positive]), typical GERD, atypical GERD, atopy (3 categories: untested, positive, negative), psychosocial disorder and FENO (categorized in > or ≤33ppb). A non-automatic backwards strategy was done so that variables with a lower level of significance were eliminated one by one. In order to evaluate the internal validity of the final model, we evaluated the discrimination as well as the calibration. The discrimination was evaluated using the area under the curve (AUC), while the Hosmer–Lemeshow test was used in order to appreciate the calibration.18 The level of significance for the contrasts was set at 0.05. The statistical software used was SPSS, version 16.0 (SPSS, Chicago, IL, USA).
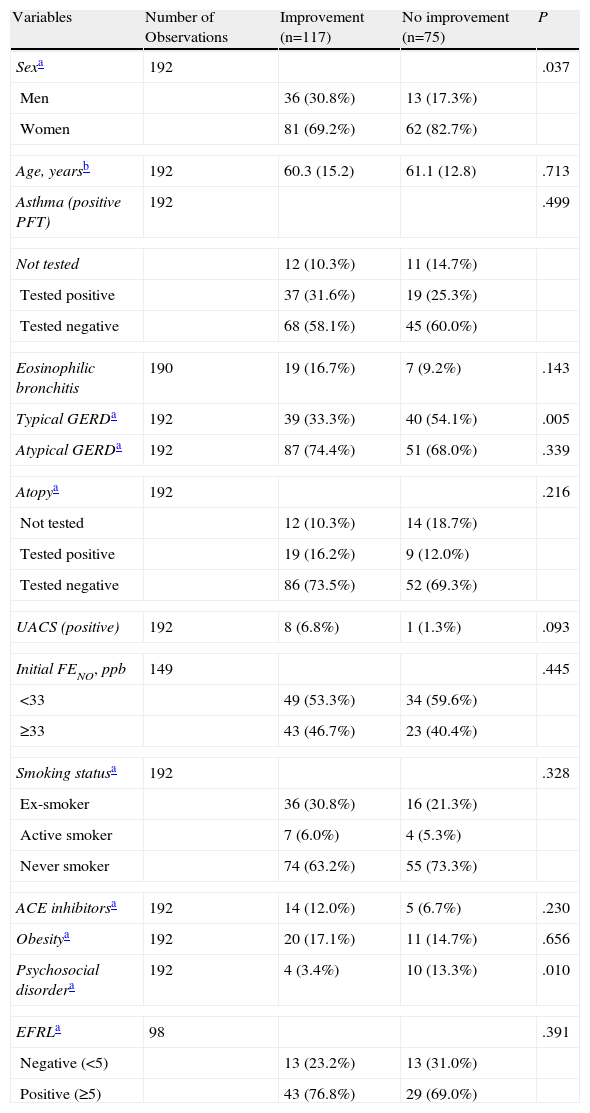
ResultsThree months later, improvement was seen in 117 cases (60.9%). Table 1 shows the results by comparing the different characteristics of the patients according to whether their cough had improved or not during the 3-month follow-up, as well as the statistical hypothesis testing with each of the variables. There were a greater proportion of women in the group who did not improve (82.7% vs 69.2%, p=.037). Also, the diagnosis of typical GERD was significantly higher in the group that did not improve (54.1% vs 33.3%, p=.005), as well as the diagnosis of individuals with psychosocial disorder (13.3% vs 3.4%, p=.010).
Characteristics of the Sample and Statistical Hypothesis Testing According to Improvement After 3 Months.
| Variables | Number of Observations | Improvement (n=117) | No improvement (n=75) | P |
| Sexa | 192 | .037 | ||
| Men | 36 (30.8%) | 13 (17.3%) | ||
| Women | 81 (69.2%) | 62 (82.7%) | ||
| Age, yearsb | 192 | 60.3 (15.2) | 61.1 (12.8) | .713 |
| Asthma (positive PFT) | 192 | .499 | ||
| Not tested | 12 (10.3%) | 11 (14.7%) | ||
| Tested positive | 37 (31.6%) | 19 (25.3%) | ||
| Tested negative | 68 (58.1%) | 45 (60.0%) | ||
| Eosinophilic bronchitis | 190 | 19 (16.7%) | 7 (9.2%) | .143 |
| Typical GERDa | 192 | 39 (33.3%) | 40 (54.1%) | .005 |
| Atypical GERDa | 192 | 87 (74.4%) | 51 (68.0%) | .339 |
| Atopya | 192 | .216 | ||
| Not tested | 12 (10.3%) | 14 (18.7%) | ||
| Tested positive | 19 (16.2%) | 9 (12.0%) | ||
| Tested negative | 86 (73.5%) | 52 (69.3%) | ||
| UACS (positive) | 192 | 8 (6.8%) | 1 (1.3%) | .093 |
| Initial FENO, ppb | 149 | .445 | ||
| <33 | 49 (53.3%) | 34 (59.6%) | ||
| ≥33 | 43 (46.7%) | 23 (40.4%) | ||
| Smoking statusa | 192 | .328 | ||
| Ex-smoker | 36 (30.8%) | 16 (21.3%) | ||
| Active smoker | 7 (6.0%) | 4 (5.3%) | ||
| Never smoker | 74 (63.2%) | 55 (73.3%) | ||
| ACE inhibitorsa | 192 | 14 (12.0%) | 5 (6.7%) | .230 |
| Obesitya | 192 | 20 (17.1%) | 11 (14.7%) | .656 |
| Psychosocial disordera | 192 | 4 (3.4%) | 10 (13.3%) | .010 |
| EFRLa | 98 | .391 | ||
| Negative (<5) | 13 (23.2%) | 13 (31.0%) | ||
| Positive (≥5) | 43 (76.8%) | 29 (69.0%) | ||
Cough variant asthma: positive PFT means that at least one of 3 tests is positive (bronchial hyperresponsiveness with methacoline, bronchodilator test or variability of peak flow).
UACS: upper airway cough syndrome.
Obesity: body mass index above 30.
EFRL: endoscopic findings of reflux in the larynx. If positive, indicates laryngopharyngeal reflux (LPR) (see explanation in the text).
GERD: gastroesophageal reflux disease.
ACE inhibitors: angiotensin-converting enzyme inhibitor.
The multiple logistic regression analysis was done with all the variables present in Table 1. The variables that provided less information in the model (p>.05) were eliminated one by one. In the end, 3 variables remained in the model: sex, typical GERD and psychosocial disorder. Being male (adjusted for psychosocial disorder and typical GERD) was associated with improvement in cough within 3 months (OR=2.10, 95%Table 2).
The data were evaluated for the existence of an interaction between sex and psychosocial disorder and sex and typical GERD, with no interaction being found. The calibration of the model was good and the frequency of the expected and observed improvements was similar (p=.409). The discrimination of the model was good, with an area under the curve of 0.663.
DiscussionIn our study, the significant response of the cough after 3 months of follow-up was identified only when the patient reported a very good visual analogical scale response or disappearance of the cough, represented by scores 3 and 4 of the scale, respectively. This is unlike other studies on chronic cough that manifest mild improvements as valid.19,20 From the analysis by sexes, we observed a significant improvement in cough (p=.037) that favored males, a fact with no clear explanation in the literature.
An improvement after 3 months was produced in 60.9% of the chronic coughers. This result is of an intermediate magnitude when compared with other series that also apply anatomical diagnostic protocols in cases with chronic cough, with percentages of satisfactory responses that range between 50% and 90%.7,19,20 These differences are probably based on the presence of aggravating factors, among which GERD was most frequent, with 79 cases of typical GERD, 138 with atypical GERD and 119 with both. This frequency of GERD is clearly higher than that in other similar studies19–21 and can be explained by the different criteria followed for its diagnosis. Typical GERD (retrosternal burning and/or regurgitation) is associated with a poorer prognosis of chronic cough. The presence of GERD can determine that, in spite of correct treatment, the cough may not be satisfactorily resolved within 6 months,22 which may justify the low success rate in our series as we only analyzed results after 3 months. Asthma-type cough was diagnosed in 56 cases out of the 165 studied (34%), a percentage similar to those of other studies.
Chronic cough can cause GERD due to the increased intra-abdominal pressured brought about by diaphragm contractions; meanwhile, the gastric content that refluxes into the esophagus can increase the cough reflex sensitivity, creating an admittedly vicious circle. Nevertheless, the literature recognizes another 3 mechanisms by which GERD can cause chronic cough: microaspiration of gastric content or reflux theory,23 the theory of the reflex mediated by the vagus nerve (resulting in the acidification of the esophagus),24 and bronchial hyperresponsiveness that produces cough, developed by the release of tachykinins as a consequence of the acid reflux in the esophagus.25 On the other hand, the precise definition of the symptoms and signs due to the extraesophageal extension of GERD is controversial. According to the conclusions of the GERD Consensus Group in Montreal, the definition of GERD is made up of esophageal and extraesophageal syndromes and, among the latter, the syndrome of chronic cough due to reflux is accepted together with the syndromes of asthma due to reflux and laryngitis due to reflux.5 We believe that the high frequency of GERD in our series is due to considering extraesophageal manifestations of GERD as valid to justify chronic cough.26 In addition, we also obviated the limitations of the exclusive use of pH-metry, because it may mean that the prevalence of non-acid or gaseous reflux is underestimated.27–29 Thus, coinciding with other authors, we consider esophageal dysmotility to be an alteration associated with chronic cough, regardless of pH-metry.30
In our opinion, there is a certain confusion in the literature by considering GERD and LPR as different clinical-pathological entities. Morice31 was one of the first authors to consider that the symptoms of LPR and GERD are two faces of the same coin, meaning that the gastric content is displaced first from the stomach to the esophagus, and later to the upper airway. This author demonstrated that there was a linear relationship between the symptoms of both entities. LPR was diagnosed in our series in 72 of 98 cases (73%) by means of endoscopic findings of reflux in the larynx, a method with sufficient validity13 as to be recommended in all studies of patients with chronic cough. Nevertheless, and according to our study, its presence does not entail a poorer prognosis of the chronic cough, probably due to the excellent response to the specific treatment.
A third factor that is present in all the series of chronic cough is rhinosinusitis, known today as upper airway cough syndrome (UACS). This syndrome was only detected in 9 cases out of 192 (0.04%), unlike the SEPAR series about the sequential treatment of chronic cough in which this aggravating factor was registered in 48% of coughers in phase I. In said series, however, the diagnosis of chronic cough was considered after the third week, which may mean that many cases could be post-viral cough with a high frequency of upper airway symptoms, the majority of which resolve themselves within 8 weeks. It is known that none of the symptoms of UACS are sufficiently sensitive or specific. Furthermore, in some patients chronic cough can be the only symptom of UACS, and it is often diagnosed after the empirical response to treatment because it is very common in the general population, lasting less than 8 weeks.32 On the other hand, conventional antihistaminic treatment aimed at rhinosinusitis can have a central cough suppressant effect, distorting specificity.
The measurement of the nitric oxide fraction in exhaled air (FENO) in the initial consultation of chronic coughers shows two clear profiles: one with levels above 33ppb and another with lower levels. In the first case, we have demonstrated in our previous experience that there is an association between chronic cough and eosinophilic inflammation of the airway, meaning asthma-type cough and/or eosinophilic bronchitis.16 In 66 of the 149 coughers analyzed, FENO was higher than 33ppb. However, the patients showed an improvement of degree 3 or 4 after 3 months without a statistically significant difference compared with patients with lower FENO, which means an excellent response to treatment with inhaled corticosteroids. What is striking is the very small incidence of smoking in this series (only 11 cases) without observing a statistically significant difference of poorer prognosis in smokers.
This study has two limitations. The first is that the analysis does not encompass all the causes of cough, but it instead focuses on those that are significant due to their frequency. Therefore, some types of cough, such as idiopathic or laryngeal neurogenic cough, were not investigated. Secondly, we did not analyze the factor of treatment compliance as there was no reliable method available to analyze this factor. Thus, this could influence the results of chronic cough, just as in any other process that requires long-term treatment.
According to the results of our study, the profile of patients with chronic cough with limited treatment response is preferentially females with typical GERD and a possible associated psychosocial disorder. To summarize, the management of chronic cough should cover numerous possibilities according to the universally used diagnostic-anatomic protocol, but it should be kept in mind that the presence of typical GERD can condition a less-than-satisfactory response to the treatment prescribed, probably due to the need for longer medication periods and strict compliance with anti-reflux techniques. Contrarily, atypical reflux and eosinophilic inflammation of the airway are not aggravating factors that condition a poorer response in chronic cough.
Conflict of InterestThere was no source of financing or conflict of interests involved in the publication of this article.
Please cite this article as: Pacheco A, et al. Tos crónica de escasa respuesta al tratamiento e incidencia de reflujo gastroesofágico. Arch Bronconeumol. 2012;48:197–201.